
Efficacy and safety analysis of selective hepatic vein occlusion combined with arterial chemoembolization versus conventional transarterial chemoembolization in the treatment of hepatocellular carcinoma
Highlight box
Key findings
• Occlusion-transarterial chemoembolization was able to achieve better complete response (CR) rates than conventional transarterial chemoembolization and did not increase the rate of corresponding adverse events.
What is known and what is new?
• Rousselot et al. and Grossi et al. demonstrated that occlusion of the hepatic venous outflow tract using a double-lumen balloon in adult mongrel dogs with simultaneous intrahepatic arterial injection of chemotherapeutic agents increased the concentration of drugs in the hepatic parenchyma, hepatic veins, lymph nodes, and thoracic ducts. Kanazawa et al., in 1993, refined this approach, employing a balloon catheter to selectively occlude the hepatic vein in swine models, which significantly enhanced the safety and reduced the complexity of the procedure.
• We obtained a better CR rate than conventional hepatic artery chemoembolization by using balloon selective occlusion of the hepatic vein followed by embolization of the tumor-supplying artery in patients with hepatocellular carcinoma.
What is the implication, and what should change now?
• This novel embolization modality achieves better local therapeutic response in patients with hepatocellular carcinoma in which the tumors being confined to the same hepatic venous drainage area.
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent cancer and the third leading cause of cancer-related mortality globally as of 2020 (1). Transarterial chemoembolization (TACE) is the most commonly used method in the treatment of unresectable HCC, demonstrating a definitive survival advantage over supportive care (2). Despite this, TACE is not curative and frequently necessitates multiple sessions to achieve desirable local tumor control. In recent years, more and more scholars have tried to update the chemotherapeutic agents, embolic materials and delivery methods to improve the efficiency of drug delivery and further improve the local control rate (3).
In the 1960s, pioneering attempts by Rousselot et al. and Grossi et al. sought to occlude the hepatic venous outflow tract using a double-lumen balloon in the inferior vena cava prior to administering chemotherapeutic agents in the hepatic artery of adult mongrel dogs. Their findings indicated that this occlusion led to increased drug concentrations within the hepatic parenchyma, hepatic veins, lymph nodes, and thoracic ducts. However, they observed a reduction in pulse pressure variation and cardiac output volume approximately 10 minutes following occlusion, indicating that the technique was relatively invasive and potentially hazardous (4,5).
Kanazawa et al., in 1993, refined this approach, employing a balloon catheter to selectively occlude the hepatic vein in swine models, which significantly enhanced the safety and reduced the complexity of the procedure. Their studies revealed that temporary segmental hepatic vein occlusion was well-tolerated for up to an hour in pigs. Perfusion imaging confirmed that this occlusion markedly increased perfusion in the occluded segments, while perfusion in the non-occluded liver segments decreased (6).
Subsequently, Hiraki et al. adapted Doppler ultrasound to monitor changes in intrahepatic hemodynamics following right hepatic vein occlusion in patients with hepatic malignancies. They observed an increase in right hepatic artery flow velocity post-occlusion, alongside a decrease in portal flow velocity. Notably, the mean peak flow velocity within the right hepatic artery dropped or stabilized between 15 to 30 seconds after occlusion, then surged and plateaued between 75 to 90 seconds, ultimately reaching 1.5 to 2 times the pre-occlusion velocity (7).
In 2007, Lee et al. attempted hepatic artery chemoembolization with temporary occlusion of the hepatic veins in patients with HCC with hepatic arteriovenous shunt. The results of their study showed that temporary balloon occlusion of the hepatic vein in HCC with hepatic artery to hepatic vein shunt allowed completion of TACE using conventional method while preventing pulmonary complications (8).
Building upon Hiraki et al.’s insights (7), we hypothesized whether extending hepatic vein occlusion duration and incrementally administering chemotherapeutic agents or embolic materials through the hepatic artery might redistribute these agents more effectively within the hepatic parenchyma and tumor, potentially surpassing the local control effect of conventional TACE (C-TACE). We have designated this novel approach as occlusion-TACE (O-TACE). Consequently, our study aims to compare the efficacy and safety profiles of O-TACE and C-TACE. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-992/rc).
Methods
Study population
This study included a total of 40 patients diagnosed with HCC in Beijing Friendship Hospital, who received initial treatment between August 2022 and March 2023. Patients were randomized into two groups, with 20 individuals each in the O-TACE and C-TACE groups.
Inclusion criteria included: (I) HCC confirmed via imaging or pathological diagnosis; (II) cases where the patient declined surgical intervention or where the tumor could not be completely resected; (III) lesions confined to the same hepatic venous drainage area, with no evidence of vascular invasion or extrahepatic metastasis; (IV) preserved liver function, categorized under Child-Pugh classes A and B; and (V) an Eastern Cooperative Oncology Group (ECOG) performance status ranging from 0 to 1. Exclusion criteria included: (I) irreversible coagulopathy; (II) current pregnancy or lactation; (III) previous targeted hepatic vein or portal vein occlusion procedures; and (IV) known allergies to iodine-based contrast agents. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Bioethics Committee of Beijing Friendship Hospital (No. 2022-P2-282-01). During the admission period of surgery, the registered patients provided written informed consent. During data collection, patient records were anonymous.
O-TACE procedure
Determination of the draining hepatic veins in the area of the lesion was based on methodologies from a previous study conducted by Murata et al. (9). Under local anesthesia, the right femoral artery and vein were accessed using the modified Seldinger technique. An 8-F guiding catheter and a 5.5-F Fogarty balloon catheter, guided by a guidewire, were inserted into the target hepatic vein to perform an angiogram. This angiogram was essential to confirm the patency of the vein, ensure the absence of stenosis, and verify that there were no significant veno-venous collateral shunts in the periphery. For the evaluation of vascular anatomy, tumor localization and extent, and portal venous flow patency, a 5-F angiography catheter was utilized to conduct angiograms of the common hepatic artery and superior mesenteric artery. Tumor-feeding arteries were then selectively catheterized using a 2.6-F microcatheter. Following the superselection, transcatheter arterial chemotherapy was administered. The protocol involved inflating the balloon, which was maintained in position for 2 minutes (Figure 1). The chemotherapy regimen selected was FOLFOX4. Concurrently, an emulsion was prepared by mixing piroxicam in a 1:2 ratio with iodized oil. The chemotherapy infusion was complemented by an embolization procedure using 300–500 μm gelatin sponge particles, which was continued until blood flow stasis was achieved. Upon completion of the embolization, the balloon was deflated, and hepatic vein patency reassessed through repeat imaging.
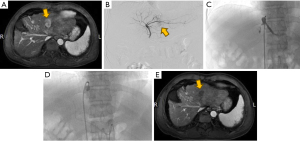
C-TACE procedure
C-TACE was performed in the absence of hepatic vein occlusion according to the same procedure as describe in section “O-TACE procedure”.
Follow-up
Adverse events (AEs) were recorded for each patient within 1 month post-procedure. These events were evaluated using the Common Terminology Criteria for Adverse Events version 4.0, focusing primarily on the identification of treatment-related adverse reactions. Biochemical parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBILI), albumin (ALB), cholinesterase (CHE), and white blood cell count (WBC), were documented before treatment and 1 month post-operatively. At the one-month mark following the initial treatment, patients underwent dynamic-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) (Figure 2). The MRI and CT scans were independently reviewed blindly by two experienced radiologists, each with more than five years of expertise. Tumor responses were evaluated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (10). A complete response (CR) was characterized by the complete disappearance of arterial enhancement in the target lesion. A partial response (PR) was defined as a reduction of greater than 30% in the sum of the diameters of viable (enhancing) lesions. Progressive disease (PD) was identified by an increase of over 20% in the sum of the diameters of viable lesions. Stable disease (SD) was determined as any instance that did not meet the criteria for PR or PD. The objective response rate (ORR) was calculated as the proportion of patients who achieved either a CR or PR. Overall survival (OS) was calculated from the commencement of TACE therapy to the date of death or loss to follow-up. Progression-free survival (PFS) was defined as the duration from the start of therapy until the progression of the liver tumor, lymph node metastases, or the emergence of distant metastasis.
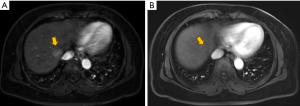
Statistical analysis
All statistical analyses were performed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Quantitative data were reported as the mean ± standard deviation (SD) and were compared between these two groups using continuity correction and the independent-samples t-test, Pearson’s χ2 test, and Fisher’s exact test. Categorical data were compared using the χ2 test. The Kaplan-Meier method and log-rank test were used to estimate and compare PFS and OS, respectively, and corresponding 95% confidence intervals (CIs) were reported. All P value calculations were two sided, and P<0.05 was considered statistically significant.
Results
Patients characteristics
In this study, a total of 40 patients were enrolled and randomly allocated into two groups: one to receive O-TACE treatment (n=20) and the other to receive C-TACE treatment (n=20). The mean age (± SD) of patients in the O-TACE group was 61.9±9.6 years, compared with 66.9±6.7 years in the C-TACE group. There were 11 (55%) and 6 (30%) patients who were chronic viral hepatitis B in the O-TACE and C-TACE groups, respectively. There were 14 (70%) cirrhotic patients in the O-TACE group compared to 11 (55%) in the C-TACE group. The median maximum tumor size of patients in the O-TACE and C-TACE groups were 3.6 and 3.2, respectively. The baseline characteristics of the two groups were compared, with no statistically significant differences observed (Table 1).
Table 1
| Characteristics | O-TACE (n=20) | C-TACE (n=20) | P value |
|---|---|---|---|
| Age, years | 61.9±9.6 | 66.9±6.7 | 0.06 |
| Sex (male/female) | 15/5 | 16/4 | >0.99 |
| HBV/none | 11/9 | 6/14 | 0.20 |
| Cirrhosis/none | 14/6 | 11/9 | 0.51 |
| PIVKA-II, mAU/mL | 119.3 [31.9, 412.6] | 275.9 [52.6, 597.3] | 0.26 |
| AFP, ng/mL | 9.0 [4.1, 329.1] | 21.5 [2.2, 207.8] | 0.86 |
| Maximum tumor size, cm | 3.6 [2.6, 6.2] | 3.2 [2.6, 6.2] | 0.87 |
| Tumor number | 1 [1, 4] | 1 [1, 1] | 0.05 |
| BCLC stage (A/B) | 12/8 | 15/5 | 0.31 |
Data are presented as mean ± standard deviation, number, or median [interquartile range]. O-TACE, occlusion-transarterial chemoembolization; C-TACE, conventional transarterial chemoembolization; HBV, hepatitis B virus; PIVKA-II, Protein Induced by Vitamin K Absence or Antagonist II; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
Tumour response and time to tumor progression
Tumor response assessment was conducted by at least two experienced radiologists using the mRECIST. At 1 month post-therapy, the CR rate was higher in the O-TACE group compared to the C-TACE group (35% vs. 5%, P=0.04). No significant differences were observed in PR rate, SD and overall response rate (ORR) between the two groups (Figure 3).

Furthermore, tumor response rates at 2 and 3 months following consecutive treatments were also evaluated. The CR rates in the O-TACE group were progressively and significantly higher than those in the C-TACE group (50% vs. 15%, P=0.04; 70% vs. 30%, P=0.02) (Figures 4,5). The levels of the liver tumor markers were notably decreased in O-TACE group, with a reduction of 48.9% (P=0.01) in alpha-fetoprotein (AFP) and 72.4% (P=0.04) in Protein Induced by Vitamin K Absence or Antagonist II (PIVKA-II). Similar but non-significant downturn was also observed in the C-TACE group (Table 2). Subgroup analysis indicated that O-TACE was a predictor of achieving a CR outcome 1 month after treatment (Table 3). Both groups showed a significant reduction in tumor diameter at 1 month post intervention compared to pre-surgical measurements (Figure 6).

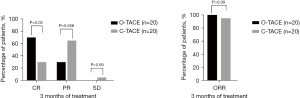
Table 2
| Parameters | O-TACE (n=20) | C-TACE (n=20) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | After | P value | Baseline | After | P value | ||
| AFP, ng/mL | 9.0 (4.1, 329.1) | 4.6 (2.7, 196.7) | 0.01 | 21.5 (2.2, 207.8) | 4.1 (1.9, 94.1) | 0.41 | |
| PIVKA-II, mAU/mL | 119.3 (31.9, 412.6) | 32.9 (11.9, 333.5) | 0.04 | 275.9 (52.6, 597.3) | 58.3 (29.1, 282.7) | 0.05 | |
Data are presented as median (interquartile range). O-TACE, occlusion-transarterial chemoembolization; C-TACE, conventional transarterial chemoembolization; AFP, alpha-fetoprotein; PIVKA-II, Protein Induced by Vitamin K Absence or Antagonist II.
Table 3
| Variables | Univariate analysis | |
|---|---|---|
| HR (95% CI) | P value | |
| Age (>60 years) | 1.462 (0.309–6.921) | 0.63 |
| Sex (male) | 0.852 (0.144–5.032) | 0.86 |
| Maximum tumor size (>3 cm) | 1.320 (0.263–6.635) | 0.73 |
| Tumor number (multiple) | 4.200 (0.459–38.445) | 0.20 |
| Cirrhosis | 0.487 (0.085–2.800) | 0.42 |
| BCLC (B) | 4.200 (0.459–38.445) | 0.20 |
| PIVKA-II (>60 mAU/mL) | 0.636 (0.110–3.694) | 0.61 |
| AFP (>600 ng/mL) | 0.764 (0.358–7.625) | 0.86 |
| O-TACE/C-TACE | 10.231 (1.121–93.341) | 0.03 |
CR, complete response; HR, hazard ratio; CI, confidence interval; BCLC, Barcelona Clinic Liver Cancer; PIVKA-II, Protein Induced by Vitamin K Absence or Antagonist II; AFP, alpha-fetoprotein; O-TACE, occlusion-transarterial chemoembolization; C-TACE, conventional transarterial chemoembolization.
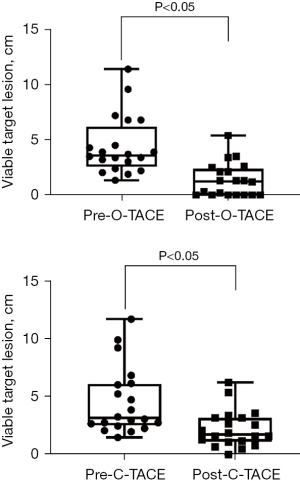
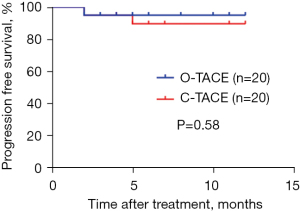
During the follow-up period, neither group reached the median PFS, and no significant difference in PFS was observed between the groups (Figure 7).
Safety analysis
Postoperative AEs were recorded for all patients, with all incidents classified as grade 1–2 AEs. Notably, no AEs associated with hepatic vein occlusion were reported. Abdominal pain was among the most prevalent AEs, occurring in 60% of patients in the O-TACE group and 65% in the C-TACE group (P=0.74). Additional AEs are listed in Table 4. Statistical analysis revealed no significant difference in the incidence of AEs between the two treatment groups.
Table 4
| AEs | O-TACE (n=20) | C-TACE (n=20) | P value |
|---|---|---|---|
| Fever | 7 (35%) | 5 (25%) | 0.49 |
| Abdominal pain | 12 (60%) | 13 (65%) | 0.74 |
| Vomiting | 4 (20%) | 3 (15%) | >0.99 |
| Fatigue | 2 (10%) | 3 (15%) | >0.99 |
AEs, adverse events; O-TACE, occlusion-transarterial chemoembolization; C-TACE, conventional transarterial chemoembolization.
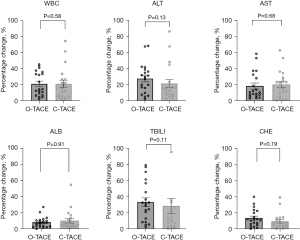
Alterations in baseline liver function indices 1 month postoperatively were also monitored. In the O-TACE group, levels of ALT, AST, and ALB were observed to be lower 1 month after the procedure compared to baseline; whereas, in the C-TACE group, only AST levels were reduced at the one-month postoperative mark in comparison to baseline values (Table 5). The levels of the liver function indices were notably decreased in O-TACE group, with a reduction of 29.0% (P=0.005) in ALT and 23.2% (P=0.02) in AST. Similar but non-significant downturn was also observed in the C-TACE group. No significant differences were detected in the comparison of individual liver function indices and leukocyte change rates post-treatment between the two patient groups (Figure 8).
Table 5
| Parameters | O-TACE (n=20) | C-TACE (n=20) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | After | P value | Baseline | After | P value | ||
| ALT, U/L | 34.5 (5.3, 46.3) | 24.5 (4.8, 36.5) | 0.005 | 20.5 (13.0, 30.8) | 15.0 (1.5, 31.3) | 0.31 | |
| AST, U/L | 36.7±17.1 | 28.2 (9.9, 36.9) | 0.02 | 29.0 (23.2, 40.2) | 24.4 (8.8, 35.7) | 0.01 | |
| TBILI, μmol/L | 21.5±10.1 | 22.5±11.6 | 0.57 | 20.9±9.1 | 19.9 (15.9, 24.9) | 0.65 | |
| ALB, g/L | 38.3±3.7 | 35.9±4.3 | 0.004 | 38.3±4.7 | 38.7±8.6 | 0.78 | |
| CHE, KU/L | 5.9±1.7 | 5.7 (4.5, 6.7) | 0.16 | 6.1±2.0 | 5.8±2.0 | 0.37 | |
| WBC, 109/L | 5.6±1.6 | 5.3±1.7 | 0.45 | 4.8±2.2 | 4.2±1.7 | 0.08 | |
Data are presented as median (interquartile range) or mean ± standard deviation. O-TACE, occlusion-transarterial chemoembolization; C-TACE, conventional transarterial chemoembolization; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBILI, total bilirubin; ALB, albumin; CHE, cholinesterase; WBC, white blood cell count.
Discussion
This study was designed to preliminarily investigate the efficacy and safety of O-TACE compared with C-TACE in patients with early to mid-stage HCC confined to a single liver lobe.
In our cohort, at 1 month postoperatively, patients in the O-TACE group achieved a CR rate of 35% vs. 5% in the C-TACE group. The ORR was comparable between the two groups, which implies that O-TACE may facilitate achieving a CR under similar conditions. Furthermore, at 2 and 3 months following consecutive treatments, the CR rate in the O-TACE group was consistently and significantly higher than in the C-TACE group. In our study the CR rate of patients in the C-TACE group was lower than that of previous studies, which may be due to the possible selective bias of fewer patients enrolled in this study on the one hand, and the inclusion of some patients with multiple nodes in this study on the other hand. In the context of recent advancements, novel embolization techniques such as balloon-assisted TACE (B-TACE) and microsphere-enhanced TACE (M-TACE) have been introduced with the aim of improving local response in HCC patients (11,12). Research by Kim et al. indicates that patients with an initial CR exhibit the longest OS, notably comparing to those who achieve a CR after multiple sessions or who attain a PR as their best outcome (13). In early to mid-stage HCC, the superior prognosis of combining TACE with ablation over either modality alone has been well-documented (14-16). A single treatment replicating the effects of combination therapy would not only alleviate patient discomfort but also lessen the financial burden, aligning with the principles of refined TACE (17,18).
Our study acknowledges that while HCC predominantly derives its blood supply from the hepatic artery, smaller tumors also receive partial supply from the portal vein, and the blood supply from hepatic artery is increasing with tumor size (19). For extracapsular invasive areas, well-differentiated tumors, and satellite nodules, the supply from the portal vein via sinusoidal pathways is crucial. Consequently, blocking only the intra-tumoral hepatic arterial flow is insufficient for complete tumor necrosis (20). Temporary occlusion of the hepatic vein raises sinusoidal pressure, potentially reducing or reversing local portal flow, thereby enhancing local drug concentration and embolization density during transarterial embolization (6). Dense iodized oil deposition, a key predictor for achieving CR post-TACE, is closely linked to tumor necrosis (21-23).
Safety-wise, the rate of AEs did not significantly differ between the two groups, and notably, there were no events related to hepatic vein occlusion in the O-TACE group. The most common AE was abdominal pain, aligning with prior studies (12,24-26). Liver function in both groups returned to baseline levels 1 month post-surgery, with the O-TACE group displaying a significant reduction in transaminases from baseline, confirming the safety of O-TACE for HCC treatment.
However, limitations of the present study include the small sample size which may impart statistical bias and the short follow-up period, where neither patient group reached median PFS or OS. Granito et al. demonstrated that it is possible to predict the local response rate of postoperative lesions by analyzing changes in transaminases after TACE. However, in our study, we only collected preoperative and 1 month postoperative transaminase changes, which did not provide a predictive value for the local response rate of the patients, and we will study the method of Granito et al. to investigate the effect of transaminases on the local response rate of the patients with O-TACE in a subsequent study (27). If we can collect serum parameters of patients before treatment, 1 week after treatment, and 1 month after treatment, we can also use line charts to describe the trend more clearly as in the study of Kim et al. (28). These aspects necessitate further exploration. Studies on the hemodynamic and molecular biological mechanisms following local hepatic vein balloon occlusion are ongoing, and the potential of this new embolization approach will be better understood once our related research series is complete.
Conclusions
O-TACE offers a superior CR rate compared to C-TACE in patients with early to mid-stage HCC limited to a single hepatic lobe, without increasing AEs. Thus, O-TACE emerges as a viable and effective treatment alternative for such HCC patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-992/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-992/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-992/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-992/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Bioethics Committee of Beijing Friendship Hospital (No. 2022-P2-282-01). During the admission period of surgery, the registered patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28-36. [Crossref] [PubMed]
- Guiu B, Chevallier P, Assenat E, et al. Idarubicin-loaded Beads for Chemoembolization of Hepatocellular Carcinoma: The IDASPHERE II Single-Arm Phase II Trial. Radiology 2019;291:801-8. [Crossref] [PubMed]
- Rousselot LM, Grossi CE, Slattery J, et al. Hepatic outflow occlusion during hepatic artery infusion with chemotherapeutic agents. Cancer 1964;17:1579-85. [Crossref] [PubMed]
- Grossi CE, Rousselot LM, Gonzalez EM, et al. Selective concentration in liver and thoracic duct lymph of anticancer drugs by hepatic outflow block. Am J Surg 1966;111:59-65. [Crossref] [PubMed]
- Kanazawa S, Wright KC, Kasi LP, et al. Preliminary experimental evaluation of temporary segmental hepatic venous occlusion: angiographic, pathologic, and scintigraphic findings. J Vasc Interv Radiol 1993;4:759-66. [Crossref] [PubMed]
- Hiraki T, Kanazawa S, Mimura H, et al. Altered hepatic hemodynamics caused by temporary occlusion of the right hepatic vein: evaluation with Doppler US in 14 patients. Radiology 2001;220:357-64. [Crossref] [PubMed]
- Lee JH, Won JH, Park SI, et al. Transcatheter arterial chemoembolization of hepatocellular carcinoma with hepatic arteriovenous shunt after temporary balloon occlusion of hepatic vein. J Vasc Interv Radiol 2007;18:377-82. [Crossref] [PubMed]
- Murata S, Itai Y, Asato M, et al. Effect of temporary occlusion of the hepatic vein on dual blood in the liver: evaluation with spiral CT. Radiology 1995;197:351-6. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Chu HH, Gwon DI, Kim GH, et al. Balloon-occluded transarterial chemoembolization versus conventional transarterial chemoembolization for the treatment of single hepatocellular carcinoma: a propensity score matching analysis. Eur Radiol 2023;33:2655-64. [Crossref] [PubMed]
- Yang Y, Du N, Ma J, et al. Efficacy and Safety of Transarterial Chemoembolization with a Three-Stage Mixed Chemoembolic Regimen for Large Unresectable Hepatocellular Carcinoma. J Hepatocell Carcinoma 2023;10:1897-910. [Crossref] [PubMed]
- Kim BK, Kim SU, Kim KA, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol 2015;62:1304-10. [Crossref] [PubMed]
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32. [Crossref] [PubMed]
- Zhang YJ, Chen MS, Chen Y, et al. Long-term Outcomes of Transcatheter Arterial Chemoembolization Combined With Radiofrequency Ablation as an Initial Treatment for Early-Stage Hepatocellular Carcinoma. JAMA Netw Open 2021;4:e2126992. [Crossref] [PubMed]
- Morimoto M, Numata K, Kondou M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010;116:5452-60. [Crossref] [PubMed]
- de Baere T, Ronot M, Chung JW, et al. Initiative on Superselective Conventional Transarterial Chemoembolization Results (INSPIRE). Cardiovasc Intervent Radiol 2022;45:1430-40. [Crossref] [PubMed]
- Wang H, Xiao W, Han Y, et al. Study on efficacy and safety of transcatheter arterial chemoembolization (TACE) combined with regorafenib and PD-1 antibody versus continued TACE combined with regorafenib in patients with hepatocellular carcinoma after failed second-line treatment with regorafenib. J Gastrointest Oncol 2022;13:1907-14. [Crossref] [PubMed]
- Ackerman NB. The blood supply of experimental liver metastases. IV. Changes in vascularity with increasing tumor growth. Surgery 1974;75:589-96. [PubMed]
- Kuroda C, Sakurai M, Monden M, et al. Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma. A study in resected cases. Cancer 1991;67:81-6. [Crossref] [PubMed]
- Park C, Gwon DI, Chu HH, et al. Correlation of tumor response on CT with pathologically proven necrosis in hepatocellular carcinoma treated by conventional transcatheter arterial chemoembolization: threshold value of intratumoral Lipiodol accumulation predicting tumor necrosis. Abdom Radiol (NY) 2021;46:3729-37. [Crossref] [PubMed]
- Dioguardi Burgio M, Ronot M, Bruno O, et al. Correlation of tumor response on computed tomography with pathological necrosis in hepatocellular carcinoma treated by chemoembolization before liver transplantation. Liver Transpl 2016;22:1491-500. [Crossref] [PubMed]
- Wang H, Xiao W, Han Y, et al. Study on safety and efficacy of regorafenib combined with transcatheter arterial chemoembolization in the treatment of advanced hepatocellular carcinoma after first-line targeted therapy. J Gastrointest Oncol 2022;13:1248-54. [Crossref] [PubMed]
- Jiang JQ, Huang JT, Zhong BY, et al. Transarterial Chemoembolization for Patients with Unresectable Hepatocellular Carcinoma with Child-Pugh B7. J Hepatocell Carcinoma 2023;10:1629-38. [Crossref] [PubMed]
- Chen R, Li L, Li Y, et al. Efficacy and safety of transcatheter arterial chemoembolization-lenvatinib sequential therapy for patients with unresectable hepatocellular carcinoma: a single-arm clinical study. J Gastrointest Oncol 2022;13:1367-75. [Crossref] [PubMed]
- Cathomas M, Mueller F, Mertineit N, et al. Comparison of transarterial bland embolization and drug-eluting beads transarterial chemoembolization for very early and early hepatocellular carcinoma not amenable for surgery or ablation: a single center retrospective data analysis. J Gastrointest Oncol 2023;14:2167-77. [Crossref] [PubMed]
- Granito A, Facciorusso A, Sacco R, et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J Pers Med 2021;11:1041. [Crossref] [PubMed]
- Kim J, Gwon DI, Kim Y, et al. Preoperative Balloon-Occluded Transcatheter Arterial Chemoembolization Followed by Surgical Resection: Pathological Evaluation of Necrosis. Diseases 2023;11:149. [Crossref] [PubMed]


