
Treatment of BRAF V600E mutant gastrointestinal stromal tumor with dabrafenib: a case report
Highlight box
Key findings
• BRAF inhibitor therapy in advanced BRAF V600E mutant gastrointestinal stromal tumor (GIST) produced durable response with minimal toxicity.
What is known and what is new?
• BRAF V600E mutant GISTs are rare and do not respond to imatinib and few case reports have shown antitumor effects of BRAF inhibitor therapy in V600E-mutant GIST.
• We report an uncommon case of BRAF V600E mutant GIST with notable tumor regression following treatment with dabrafenib.
What is the implication, and what should change now?
• Molecular testing should be considered to guide treatment decision making in GIST.
• Treatment with BRAF inhibitors should be considered front line therapy in patients advanced BRAF V600E mutant GIST.
Introduction
Gastrointestinal stromal tumor (GIST) is a rare mesenchymal tumor that most frequently arises in the stomach or small bowel. The most common driver mutation underlying GIST pathogenesis is a KIT (~80%) mutation which promotes tumorigenesis via constitutive activation of tyrosine kinase receptors (1). The second most common, are PDGFRA (~8%) mutations which are rare and often imatinib resistant (1). First line therapy with imatinib (a KIT/PDGFRA inhibitor) produces high response rates in cases of advanced GIST with imatinib-sensitive KIT or PDGFRA mutant kinases (2). However, approximately 15% of GISTs lack an underlying KIT or PDGFRA mutation, and these types of GIST have minimal to no response to imatinib (1-3). BRAF V600E mutant GIST is a rare GIST subtype. In cBioPortal, there are sequencing results for 533 unique GIST cases. BRAF V600E mutations were found in 3 of these cases (0.6%). This frequency is consistent with published estimates based on the review of multiple reported series. For example, Khosroyani et al. estimated a frequency of 0.8% (3,4). Due to the rarity of this type of GIST, there are few reports describing treatment of this molecular subtype. BRAF mutations have been found in multiple other malignancies, including cutaneous melanoma, colorectal carcinoma, and thyroid cancer although notably colorectal cancers with this mutation are not been responsive to BRAF inhibitors (5). Dabrafenib is a selective inhibitor of the BRAF kinase, and has shown therapeutic efficacy in BRAF-mutant cancers, with superior results when combined with the MEK inhibitor, trametinib (3,6,7). Multiple clinical trials have demonstrated the antitumor effects of dabrafenib in BRAF-mutant melanoma, however, there are only two case reports of treatment of BRAF V600E mutant GIST with a BRAF kinase inhibitor, although both reports are for the same patient, albeit detailing different points in the treatment history (8,9). The following case describes the anti-tumor effects of dabrafenib in a patient with BRAF V600E mutant GIST. We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-767/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). This study was conducted under local institutional review board of Oregon Health and Science University (protocol 24977). For this retrospective chart review study, the institutional review board granted a waiver of written informed consent for the publication of this case report and accompanying images.
A 67-year-old woman initially presented with abdominal pain. Physical exam revealed a palpable lesion in her left mid abdomen, and abdominal computed tomography (CT) scan confirmed a 14 cm × 13 cm mass suggestive of an ovarian primary; however, the patient had previously had bilateral oophorectomy. She underwent surgical resection of the mass which was ultimately diagnosed as a GIST, mixed epithelioid and spindled type. Immunohistochemistry (IHC) was positive for DOG-1, CD117 and caldesmon. IHC for CD10, CD34, HMB45, smooth muscle actin, S-100 and desmin were negative. Pathologic examination identified a high-risk tumor arising from the small bowel with 50 mitoses per 50 high-powered fields. She was started on adjuvant imatinib; however, when molecular analysis (next generation sequencing) of the tumor revealed an absence of KIT or PDGFRA mutations, therapy was discontinued after six months and she was followed with active surveillance only (10). It was however notable for a BRAF V600E, PTCH1 A1337_G1343del, MEN1 Q141*, and TERT promoter −124C>T mutations. Approximately 8 months after her initial resection, imaging identified metastatic disease. Based on an initial diagnosis of a “wild-type” GIST she was treated with sunitinib, but her treatment was complicated by significant hand-foot skin reaction, as well as early disease progression. To guide future treatment, her initial tumor resection specimen underwent additional molecular analysis, which identified a BRAF V600E mutation. The patient was started on third-line regorafenib approximately 3 months after discontinuation of sunitinib, but this treatment was again complicated by severe hand-foot skin reaction and mucositis. She was hospitalized with sepsis secondary to community-acquired pneumonia within the same month of regorafenib initiation, leading to discontinuation of this therapy.
Following treatment of her pneumonia and recovery from her regorafenib side effects, she began treatment with the BRAF inhibitor dabrafenib, dosed at 150 mg twice a day, based on a prior case report (9). During her initial three months of treatment, she noticed decreasing size, and then resolution, of her palpable abdominal wall masses. Sensation of urinary urgency due to bladder compression from pelvic masses also resolved. During this time, she reported no significant side effects from dabrafenib. Notably, her imaging revealed a dramatic response with significant tumor shrinkage, including multiple lesions with more than a 50% decrease in longest diameter (Figures 1,2). She was evaluated for surgical intervention, but given the multifocal nature of her metastases, as well as her age, surgery was not recommended. She continued single agent dabrafenib with ongoing partial response, and with no evidence of cardiomyopathy, thyroid dysfunction, or renal, hepatic, or bone marrow toxicity.
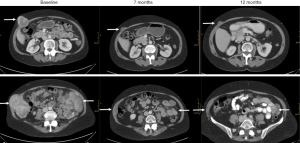

A partial imaging response was obtained at 7 months and after about 12 months of single agent dabrafenib, her imaging showed some tumor progression, but overall, her disease burden at that time was still significantly less than her pre-dabrafenib baseline (Figure 1). Her adherence to the medication was assessed and determined to be very good, although she reported some missed doses. Based on data from metastatic melanoma treatment studies, she was then started on dual therapy with dabrafenib 150 mg twice a day and trametinib 2 mg daily. She continued combination dabrafenib and trametinib for approximately 3 months with good tolerance of the combination treatment, but began to notice some abdominal fullness on her right side. Imaging at that time revealed continued progression on dual therapy, although some lesions remained stable.
Based on extrapolation from studies using treatment of BRAFi/MEKi resistant melanoma and a prior phase 1/2 study of mammalian target of rapamycin (mTOR) inhibitor everolimus for imatinib-resistant GIST (9,11), she was switched to single agent therapy with everolimus 10 mg a day. Follow up imaging showed a mixed response with overall stability, with shrinkage of some tumors but growth of others (Figure 1). After about 4 months of everolimus she noted more awareness of her abdominal masses, and imaging confirmed progression of her disease. A trial of rechallenge with BRAF + MEK inhibition was initiated, based on evidence of switching back and forth from BRAF/MEK inhibitors to PI3K-AKT inhibitors, then back to BRAF/MEK inhibitors in melanoma. Everolimus was stopped, and she was restarted on dabrafenib 150 mg twice a day and trametinib 2 mg daily. Imaging after 2 months of combination therapy showed a mixed response, with some tumors decreasing, some with mild increases in size, and others with marked density changes indicative of response. Her labs showed normal renal and hepatic function, and a stable hemoglobin of 8.4 with no leukopenia or thrombocytopenia. After 5 months of rechallenge with dabrafenib and trametinib, imaging revealed that her lesions were markedly necrotic and had coalesced into a septated, very large abdominal mass—26.6 cm × 12.4 cm × 23.3 cm. Over the next few weeks, she developed gastrointestinal (GI) bleeding due to a duodenal ulcer and biopsy confirmed esophageal candidiasis. She was discharged to a skilled nursing facility for higher level of care. Unfortunately, she became progressively weaker, and was transitioned to hospice care and subsequently passed away.
Discussion
This patient presented with an uncommon molecular subtype of GIST that was hypothesized to be initiated and sustained by the BRAF V600E mutation (12). Activating BRAF V600E mutations are hypothesized to be the initial driver mutation in approximately 0.8% of GISTs (4). This subtype most commonly arises in the small intestine due to excessive activation of the MEK-ERK signaling pathway (12). In addition, two separate groups have reported that transgenic mice with BRAF V600E mutations targeted to GIST precursor cells develop GIST-like tumors (13,14). In the report by Ran et al. (14), targeting BRAF mutation to ETV1+ interstitial cells of Cajal (ICC) cells resulted in ICC hyperplasia, with inactivation of Trp53 required for development of malignant tumors. In the report by Kondo et al., targeting BRAF V600E using Myh11 resulted in ICC hyperplasia and GIST-like tumor formation (12). In this model, smooth muscle precursor cells rather than ICC seemed to be the cell of origin, with ICC hyperplasia arising from smooth muscle precursor cells.
Given BRAF V600E as the hypothesized driver mutation, the patient’s disease would be predicted to demonstrate resistance to standard GIST therapy with KIT inhibitors, as exemplified by this case—there was no evidence of tumor regression when the patient was on non-BRAF inhibitors, but treatment with dabrafenib resulted in notable tumor regression. Her course was complicated by eventual disease progression due to acquired resistance.
Based on previous trials investigating the most optimal combination drug therapies for BRAF-mutant melanoma, it has been speculated that adding a MEK inhibitor such as trametinib to a BRAF inhibitor regimen may be a more optimized treatment for BRAF-mutant GIST. While single agent BRAF inhibitors such as dabrafenib are active against patients with BRAF V600E mutant melanoma, duration of response is limited due to acquired resistance. Studies suggest that a combination of inhibitors of BRAF as well as MEK can potentially decrease development of acquired resistance driven by the mitogen-activated protein kinase (MAPK) pathway. Indeed, randomized studies of BRAF inhibitors versus a combination of the same inhibitor with an MEK inhibitor have shown improved treatment outcomes [e.g., progression-free survival (PFS), overall survival (OS)] (15,16). As shown in Figures 1,2, our patient has a Response Evaluation Criteria of Solid Tumors (RECIST) partial response to single agent dabrafenib, with a maximal tumor shrinkage of 63% and was treated for almost 500 days before progression was noted.
Subsequent to our treatment of this patient, in the summer of 2022, the Food and Drug Administration (FDA) granted accelerated approval to the combination of dabrafenib and trametinib for treatment of adult and pediatric BRAF V600E mutant solid tumor after progression on prior treatment and for whom no satisfactory treatment options were available (17). Previously, the combination has been approved for treatment of unresectable or metastatic BRAF V600E mutant melanoma, anaplastic thyroid cancer, and non-small cell lung cancer. This most recent approval was based on the results from the open-label phase 2 basket studies NCT02024110 (ROAR) (18), NCT02465060 (NCI-MATCH cohort H) (19), and NCT04507919 (CTMT212X2101), and supported by results from COMBI-d, COMBI-v, TAPUR and BRF113928 (20,21). Notably, in the ROAR, NCI-Match cohort H studies, only a single patient with GIST was treated, and this patient did not have an objective response endpoint reported although did have stable disease for 30 months on dabrafenib and trametinib (5,18,19). However, based on the pre-specified mutation specific/tissue agnostic study design, dabrafenib and trametinib were approved for treatment of BRAF V600E mutant solid tumors, which includes BRAF V600E mutant GIST.
A previous case of BRAF V600E mutant GIST demonstrated that one of the mechanisms underlying acquired resistance was an acquired gain-of-function PIK3CA mutation (8,9). The melanoma literature also suggests that there may be a role for rechallenge with BRAF and MEK inhibitors in the setting of progression. For example, a phase II clinical trial evaluating the use of dabrafenib plus trametinib in BRAF V600-mutant melanoma patients found that those who demonstrated disease progression on BRAF (+MEK)-inhibitors who were off BRAF (+MEK) inhibitor therapy for at least 12 weeks and progressed on immunotherapy had meaningful benefit from rechallenge (22). These data formed the basis for our decision to initiate interim treatment with everolimus, an inhibitor of the PI3K-AKT-mTOR pathway, and later to rechallenge with dabrafenib/trametinib which yielded a transient response before fatal tumor progression.
Conclusions
Our case highlights the importance of molecular testing in GIST to guide treatment decision making, as the patient received three lines of KIT directed therapy (imatinib, sunitinib, and regorafenib) with toxicity but no benefit. In contrast, BRAF inhibitor therapy produced a durable response with minimal/acceptable toxicity before secondary resistance developed. Based on a recent FDA approval, patients with advanced BRAF V600E mutant GIST should now be considered for front-line therapy with the combination of dabrafenib and trametinib. However, given the limited treatment outcome data for such patients, it remains important to continue to collect data on such patients to help verify the efficacy of this treatment for advanced BRAF-mutant GIST.
Acknowledgments
Funding: The study was supported by a research grant from
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-767/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-767/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-767/coif). M.C.H. received partial salary support from the following sources: a research grant from the Jonathan David Foundation, a VA Merit Review Grant (No. I01BX005358), and from NCI R21 grant (No. R21CA263400). He also receives consulting fees from Novartis, Deciphera Pharmaceuticals, Blueprint Medicines, Cogent Pharmaceuticals, Cstone Pharmaceuticals, Theseus Pharmaceuticals, and New Bay Pharmaceuticals. A patient on the treatment of GIST with imatinib was licensed by M.C.H.’s institution to Novartis. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). This study was conducted under local institutional review board of Oregon Health and Science University (protocol 24977). For this retrospective chart review study, the institutional review board granted a waiver of the need of written informed consent for the publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in "Wild-Type" gastrointestinal stromal tumors. J Transl Med 2016;14:339. [Crossref] [PubMed]
- Blay JY, Kang YK, Nishida T, et al. Gastrointestinal stromal tumours. Nat Rev Dis Primers 2021;7:22. [Crossref] [PubMed]
- Khosroyani HM, Klug LR, Heinrich MC. TKI Treatment Sequencing in Advanced Gastrointestinal Stromal Tumors. Drugs 2023;83:55-73. [Crossref] [PubMed]
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401-4. [Crossref] [PubMed]
- Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med 2023;29:1103-12. [Crossref] [PubMed]
- Halle BR, Johnson DB. Defining and Targeting BRAF Mutations in Solid Tumors. Curr Treat Options Oncol 2021;22:30. [Crossref] [PubMed]
- Corrie P, Meyer N, Berardi R, et al. Comparative efficacy and safety of targeted therapies for BRAF-mutant unresectable or metastatic melanoma: Results from a systematic literature review and a network meta-analysis. Cancer Treat Rev 2022;110:102463. [Crossref] [PubMed]
- Kato S, Adashek JJ, Shaya J, et al. Concomitant MEK and Cyclin Gene Alterations: Implications for Response to Targeted Therapeutics. Clin Cancer Res 2021;27:2792-7. [Crossref] [PubMed]
- Falchook GS, Trent JC, Heinrich MC, et al. BRAF mutant gastrointestinal stromal tumor: first report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget 2013;4:310-5. [Crossref] [PubMed]
- Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial. JAMA Oncol 2017;3:602-9. [Crossref] [PubMed]
- Schöffski P, Reichardt P, Blay JY, et al. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Ann Oncol 2010;21:1990-8. [Crossref] [PubMed]
- Kondo J, Huh WJ, Franklin JL, et al. A smooth muscle-derived, Braf-driven mouse model of gastrointestinal stromal tumor (GIST): evidence for an alternative GIST cell-of-origin. J Pathol 2020;252:441-50. [Crossref] [PubMed]
- Xie Y, Cao Z, Wong EW, et al. COP1/DET1/ETS axis regulates ERK transcriptome and sensitivity to MAPK inhibitors. J Clin Invest 2018;128:1442-57. [Crossref] [PubMed]
- Ran L, Murphy D, Sher J, et al. ETV1-Positive Cells Give Rise to BRAF(V600E) -Mutant Gastrointestinal Stromal Tumors. Cancer Res 2017;77:3758-65. [Crossref] [PubMed]
- Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 2017;28:1631-9. [Crossref] [PubMed]
- Ascierto PA, Dummer R, Gogas HJ, et al. Update on tolerability and overall survival in COLUMBUS: landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer 2020;126:33-44. [Crossref] [PubMed]
- FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid
- Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol 2022;33:406-15. [Crossref] [PubMed]
- Salama AKS, Li S, Macrae ER, et al. Dabrafenib and Trametinib in Patients With Tumors With BRAF(V600E) Mutations: Results of the NCI-MATCH Trial Subprotocol H. J Clin Oncol 2020;38:3895-904. [Crossref] [PubMed]
- Schadendorf D, Long GV, Stroiakovski D, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 2017;82:45-55. [Crossref] [PubMed]
- Meric-Bernstam F, Rothe M, Mangat PK, et al. Cobimetinib Plus Vemurafenib in Patients With Solid Tumors With BRAF Mutations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis Oncol 2023;7:e2300385. [Crossref] [PubMed]
- Schreuer M, Jansen Y, Planken S, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAF(V600)-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol 2017;18:464-72. [Crossref] [PubMed]


