
Transarterial chemoembolization for omental vein tumor thrombosis in hepatocellular carcinoma: a case report
Highlight box
Key findings
• A case of omental vein tumor thrombosis (OVTT) in a hepatocellular carcinoma (HCC) patient treated with transarterial chemoembolization (TACE).
What is known and what is new?
• In HCC patients: vascular invasions such as portal vein tumor thrombosis (PVTT) and hepatic vein tumor thrombosis is crucial for disease staging.
• This represents the first documented case of TACE for OVTT in HCC in the medical literature, highlighting a rare but significant pattern of vascular invasion in HCC.
What is the implication, and what should change now?
• This case report highlights the uncommon presentation of OVTT alongside PVTT in advanced HCC, emphasizing the need for vigilance in diagnosing atypical disease progressions in HCC and a possible treatment efficacy of TACE.
Introduction
In the clinical landscape of hepatocellular carcinoma (HCC), the identification of macrovascular invasion, particularly portal vein tumor thrombosis (PVTT) and to a lesser extent, hepatic vein tumor thrombosis (HVTT), has become a critical determinant in classifying the stage of disease (1). Such macrovascular invasions are not only prognostically significant, indicating a grave outlook for overall patient survival but also requiring substantial alterations in the management of HCC. The presence of macrovascular invasion signifies that the patient is no longer a candidate for curative treatment modalities such as surgical resection, liver transplantation, or radiofrequency ablation (RFA) (2).
The incidence of PVTT varies widely, occurring in 6.5% to 44% of HCC patients, whereas HVTT alone is seen in only 3% (3). According to Mähringer-Kunz et al.’s study, HVTT occurs concomitantly with PVTT in 26.9% of cases (4). The guidelines from the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases currently recognize tumor thrombosis primarily in the portal vein (1,5). Nonetheless, the presence of tumor thrombosis in veins other than the portal vein is also pivotal, as it reclassifies the staging of the disease (2). Such cases of macrovascular invasion involving non-portal and non-hepatic veins remain scant in medical literature.
We report a patient with recurrent HCC patient who was presented with omental vein tumor thrombosis (OVTT) 15 years after receiving RFA therapy and was treated with conventional transarterial chemoembolization (TACE). We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-17/rc).
Case presentation
A 74-year-old male patient with a history of hepatitis B was previously diagnosed with solitary HCC in segment 8, which was treated with RFA 15 years ago. He had been managed for hepatitis B with Tenofovir alafenamide (25 mg/day) for the past 10 years and was regularly monitored for HCC tumor markers. During 3 months prior to hospitalization, his serum alpha-fetoprotein (AFP) levels gradually increased from 159 to 247 ng/mL.
Upon admission, the patient was in good general condition with mild right upper quadrant abdominal pain. His performance status was grade 1 according to the Eastern Cooperative Oncology Group Criteria, and he had a Child-Pugh score of six points. Basic laboratory tests revealed: 10.6 g/L hemoglobin, 3.7 g/dL albumin, 0.45 mg/dL bilirubin, 0.08 mg/L C-reactive protein (CRP), 58 U/L aspartate transaminase (AST), and 57 U/L alanine transaminase (ALT). Red blood cell (RBC) counts were 4.3 g/L, 2.48 g/L white blood cells (WBC) with 53.3% neutrophils, and 77 g/L platelets. HCC markers included AFP at 2,127 ng/mL and prothrombin induced by vitamin K absence-II (PIVKA-II) at 22 pg/mL.
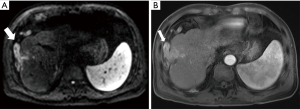
Magnetic resonance imaging (MRI) with contrast enhancement demonstrated the recurrence of HCC in segment 8 at the previously treated site by RFA, with the recurrent tumor measuring 25 mm × 30 mm. This lesion presented ill-defined margins with the surrounding normal liver parenchyma and showed significant contrast uptake in the arterial phase followed by washout in the venous and delayed phases, which is characteristic of HCC. The lesion also caused a thrombosis in a branch of the portal vein adjacent to the tumor. Anterior to the tumor, there was an omental vein with unclear boundaries with the tumor, measuring 18 mm in diameter, demonstrating restricted diffusion on diffusion-weighted imaging (DWI), strong contrast enhancement in the arterial phase, and washout in both the venous and delayed phases, indicating tumor invasion (Figure 1).
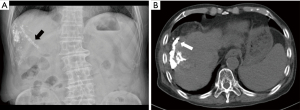
The patient underwent conventional TACE twice using 5 mL of ethiodized oil (Lipiodol, Guerbet, Roissy, France) mixed with 30 mg of doxorubicin (Ildong, Seoul, Korea), one month apart. Digital subtraction angiography revealed hypervascularization of the tumor and tumor thrombosis supplied by branches of the right hepatic artery and omental artery. The OVTT was vascularized by branches of the omental artery from the right gastroepiploic artery (Figure 2). The procedure was performed using cone-beam computed tomography (CT) imaging with a 1.8 Fr microcatheter (Progreat, Terumo, Tokyo, Japan), guided by Emboguide software (Syngo Embolization Guidance, Siemens, Frankenthal, Germany), to access and infuse the doxorubicin and ethiodized oil mixture into the tumor-feeding vessels. The vessels were then embolized using 150–350 µm-sized gelatin microparticles (EG gel, Engain, Gyeonggi, Republic of Korea). Abdominal X-ray immediately after TACE confirmed lipiodol accumulation at the tumor site and OVTT. After the first procedure, the patient’s AST and ALT serum levels increased to 587 and 402 U/L, and after the second procedure, they increased to 520 and 428 U/L. The elevation of AST and ALT recovered within 3 days after conservative treatment and the patient was discharged. There were no other complications related to TACE. A follow-up CT scan three months post-TACE showed no remaining viable tumor, with a marked decrease in tumor markers, AFP at 26.1 ng/mL and PIVKA-II at 5 pg/mL (Figure 3).

The study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea (Approval No. KC23ZISI0847). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
HCC is a malignancy that characteristically vascular invasion, particularly the portal and hepatic veins, with a minority of cases reported to extend into the biliary tract (6). Concurrently, the presence of extrahepatic collateral (EHC) blood supply is relatively common, especially in large tumors or recurrent HCCs (7). The omental artery is the second or third most common EHC artery providing blood supply to HCCs, accounting for approximately 13% of cases, with a tendency to vascularize subcapsular tumor locations. In the case presented, the tumor received additional blood supply from a prominent omental artery beyond the feeding arteries from the right hepatic artery, suggesting that the omental arterial supply may be a contributing factor for the emergence of tumor thrombosis within the omental vein (7). However, contrary to the identification of extrahepatic arterial branches supplying HCC to optimize TACE efficacy for maximal tumor response, the occurrence of tumor thrombosis in veins other than the portal and hepatic veins has not been prevalently documented in the literature (7). There have been reports of unusual venous lesions unrelated to HCC in the omental vein (8,9). In patients with cirrhosis, collateral circulation may develop between the portal and systemic venous systems in response to portal hypertension: connections between the superior and inferior mesenteric veins, between the omental vein and the retroperitoneal or pelvic veins, and potentially other anastomoses involving the gastroepiploic veins (10,11).
In our patient, tumor recurrence was observed eight years after treatment with RFA. RFA is extensively utilized for early and very early stages of HCC, achieving survival rates comparable to those of liver resection (12,13). Late recurrence is defined as recurrence occurring more than two years of curative treatment, with a late recurrence rate reported by Yang et al. as 24.6%, of which local recurrences accounted for 14.3% (14). In our case, the tumor exhibited localized recurrence at the RFA treatment site alongside localized vascular invasion, including OVTT and PVTT. Yang et al. also identified male gender, the presence of multiple liver tumors, and cirrhosis as risk factors for late recurrence (14). Stabilizing hepatitis B infection is a critical factor in reducing the risk of HCC tumor recurrence (13).
Recent advancements in the treatment of advanced-stage HCC with vascular invasion have significantly evolved with the advent of new systemic therapy agents (2). Combination therapy with atezolizumab and bevacizumab (AB) is considered the first-line option in most current guidelines, Barcelona Clinic Liver Cancer (BCLC) 2022, yet the guideline does not widely acknowledge vascular invasion beyond the portal vein, including HVTT (15,16). Based on the IMbrave 150 study, the AB combination has shown superior efficacy compared to sorafenib in patients with HCC, achieving an objective response rate (ORR) of 30%. However, treatment-related grade 3/4 adverse events were observed in 143 (43%) of 329 patients. The effect of the AB combination on patients with localized progression and macrovascular invasion is not yet fully elucidated, as 207 out of 336 patients (61.6%) did not present with macrovascular invasion (17). Meanwhile, for HCC cases with localized progression, locoregional therapies such as TACE or hepatic arterial infusion chemotherapy (HAIC) have been demonstrated to achieve optimal tumor response (16). Therefore, we opted for TACE treatment for patients even in the advanced stages. However, to achieve optimal tumor response and provide the greatest survival benefit, combination with systemic therapy is necessary (16).
The selection of TACE for patients with HCC and segmental PVTT is judiciously considered in patients with preserved liver function, Child-Pugh A or B, showing a high tumor response rate, especially in cases of hypervascular PVTT (18). In our patient, Lipiodol uptake was observed in both PVTT and OVTT after conventional TACE. AST and ALT elevated after TACE. This is a common TACE-related complication. Post-treatment transient AST and ALT elevation was predictive of objective response to TACE (19). In Asian clinical guidelines and practice, TACE is regarded as a more commonly chosen alternative treatment option for BCLC stage C patients compared to sorafenib in previous stages (20). The integration of systemic treatments or other local modalities such as stereotactic body radiotherapy or HAIC can provide maximal tumor response benefits and extend patient survival (20).
Conclusions
This case report highlights the uncommon presentation of OVTT alongside PVTT in advanced HCC, emphasizing the need for vigilance in diagnosing atypical disease progressions in HCC and a possible treatment efficacy of TACE.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-17/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-17/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Institutional Review Board of Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea (Approval No. KC23ZISI0847). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Khan AR, Wei X, Xu X. Portal Vein Tumor Thrombosis and Hepatocellular Carcinoma - The Changing Tides. J Hepatocell Carcinoma 2021;8:1089-115. [Crossref] [PubMed]
- Kim JH, Lee JM, Yoon JH, et al. Portal Vein Thrombosis in Patients with Hepatocellular Carcinoma: Diagnostic Accuracy of Gadoxetic Acid-enhanced MR Imaging. Radiology 2016;279:773-83. [Crossref] [PubMed]
- Mähringer-Kunz A, Meyer FI, Hahn F, et al. Hepatic vein tumor thrombosis in patients with hepatocellular carcinoma: Prevalence and clinical significance. United European Gastroenterol J 2021;9:590-7. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- Wang C, Yang Y, Sun D, et al. Prognosis of hepatocellular carcinoma patients with bile duct tumor thrombus after hepatic resection or liver transplantation in Asian populations: A meta-analysis. PLoS One 2017;12:e0176827. [Crossref] [PubMed]
- Moustafa AS, Abdel Aal AK, Ertel N, et al. Chemoembolization of Hepatocellular Carcinoma with Extrahepatic Collateral Blood Supply: Anatomic and Technical Considerations. Radiographics 2017;37:963-77. [Crossref] [PubMed]
- Fonseca AL, Cleary MA, Cholewczynski W, et al. Omental vein catheter thrombolysis for acute porto-mesenteric vein thrombosis. Ann Vasc Surg 2013;27:497.e1-4. [Crossref] [PubMed]
- Sanomura T, Norikane T, Uchinomura S, et al. Omental arteriovenous fistula after splenectomy treated with transarterial embolization. CVIR Endovasc 2023;6:28. [Crossref] [PubMed]
- Ito K, Fujita T, Shimizu A, et al. Imaging findings of unusual intra- and extrahepatic portosystemic collaterals. Clin Radiol 2009;64:200-7. [Crossref] [PubMed]
- Sharma M, Rameshbabu CS. Collateral pathways in portal hypertension. J Clin Exp Hepatol 2012;2:338-52. [Crossref] [PubMed]
- Chen R, Hou B, Zhou Y, et al. Recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: Analysis of the pattern and risk factors. Front Oncol 2023;13:1018715. [Crossref] [PubMed]
- Xin Y, Zhang X, Yang Y, et al. Prediction of late recurrence after radiofrequency ablation of HBV-related hepatocellular carcinoma with the age-male-albumin-bilirubin-platelets (aMAP) risk score: a multicenter study. J Gastrointest Oncol 2021;12:2930-42. [Crossref] [PubMed]
- Yang Y, Chen Y, Ye F, et al. Late recurrence of hepatocellular carcinoma after radiofrequency ablation: a multicenter study of risk factors, patterns, and survival. Eur Radiol 2021;31:3053-64. [Crossref] [PubMed]
- Stefanini B, Ielasi L, Chen R, et al. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 2023;23:279-91. [Crossref] [PubMed]
- Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2023;20:203-22. [Crossref] [PubMed]
- Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-73. [Crossref] [PubMed]
- Silva JP, Berger NG, Tsai S, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford) 2017;19:659-66. [Crossref] [PubMed]
- Granito A, Facciorusso A, Sacco R, et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J Pers Med 2021;11:1041. [Crossref] [PubMed]
- 2022 KLCA-NCC Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol 2022;23:1126-240. [Crossref] [PubMed]


