Cytoreductive surgery and intraperitoneal chemotherapy: an evidence-based review—past, present and future
Introduction
Peritoneal carcinomatosis (PC) is defined as tumor dissemination inside the peritoneal cavity arising from primary peritoneal malignant disorders (such as peritoneal mesothelioma) and digestive-tract and gynecological advanced cancers [such as appendiceal tumor, ovarian cancer (OC), colorectal cancer (CRC), or gastric cancer] (1). PC has historically been considered a terminal condition treated with palliative means that included supportive care, palliative surgery and systemic chemotherapy achieving survival rates measured only in months (2). Fortunately, in the 1980s, renewed interest in malignant diseases with peritoneal extension and the introduction of the concept of a ‘locoregional disease’ resulted in the birth of a new approach. Cytoreductive surgery (CyRS) and intraperitoneal chemotherapy (IPC) emerged as possibly more effective treatments with a potential for long term survival. Although preliminary data were viewed with great skepticism, to date, this strategy is the only one that has been associated with prolonged survival with median overall survival (OS) as long as 46 months (3).
PC is presumed to develop via shedding of malignant cells from the primary tumor after breaching the peritoneal lining of the organ of origin. It has been postulated that peritoneal metastases may represent regional disease, and that cancer cells may not have spread any further throughout the body (4). This concept known as ‘regional metastases’ to the peritoneum has been gradually embraced by multiple groups over the past two decades; and as a result, aggressive management of peritoneal surface malignancy with CyRS and IPC is now widely practiced. However, data from randomized controlled trials (RCTs) evaluating the efficacy of this aggressive therapy in different tumor pathology are scarce (5).
CyRS, in combination with perioperative IPC, was first described by Sugarbaker in the 1990s as an aggressive form of ‘locoregional’ therapy (6,7). The fundamentals of this technique as well as a variety of other aspects are described in separate chapters of this review. Briefly, the goal of CyRS is to eradicate the peritoneal cavity of any gross disease. This can be followed by infusion of a chemotherapeutic agent either during the operation or in the early post-operative period. This process allows higher concentrations of the drug within the peritoneum where the cancer tends to recur. Based on this strategy, CyRS in conjunction with IPC is believed to obtain macroscopic and microscopic clearance and therefore may improve survival (8). The aim of this review is to highlight cancer-specific evidence in the context of current and future studies regarding the outcome of this treatment.
The state of the current literature as a basis for modern trials
Over the last two decades, a growing body of evidence has accumulated in support of CyRS and IPC as a treatment modality for PC. Consequently, centers across the world have adopted this modality contributing to the exponential rise in the popularity of this procedure and thus the supporting literature. This expanding literature has helped identify the types of cancers for which CyRS and hyperthermic intraperitoneal chemotherapy (HIPEC) may be associated with survival benefit. Best results with CyRS and IPC have been seen with appendiceal, colorectal, ovarian, and primary peritoneal cancer.
However, peritoneal metastasis can be seen with histologic subtypes that do not usually metastasize to the abdominal cavity such as breast cancer, melanoma, neuroendocrine tumors (NETs), and sarcoma. Therefore, there have been attempts to use this modality to treat abdominal metastasis associated with those cancers. No clear guidelines are available regarding the role of CyRS with or without IPC in PC from pancreatic, primary hepatic, breast cancer and melanoma, nor does the literature provide reliable information on these patients’ prognosis, as most papers consist of case reports and small case series (9-12). In contrast, there are some data in the literature regarding the efficacy of CyRS and IPC in NETs and sarcoma. Elias et al., have recently reported their experience using CyRS and HIPEC in treating 41 patients with NET peritoneal metastases. They concluded that CyRS of peritoneal metastases from a NET is feasible and may be associated with better survival. However, they were unable to unable to determine whether adding HIPEC had any impact (13). Similarly, IPC has not been shown to improve survival in patients with peritoneal metastases from sarcomas including gastrointestinal stromal tumor (GIST) (14,15) or desmoplastic small round-cell tumors (16). Therefore, based on the current literature, IPC is currently recommended for appendiceal, colorectal, ovarian, and primary peritoneal cancer, and, to a lesser extent, gastric cancer. The quality of the evidence supporting the use of CyRS and IPC in each of these cancers varies from one tumor entity to another and evidence from randomized trials is often lacking. The literature regarding the outcomes of CyRS and IPC will be, therefore, discussed separately for each of these tumor types.
Appendiceal mucinous neoplasms
Appendix neoplasms are rare intraperitoneal tumors arising primarily from an appendiceal epithelial neoplasm. Appendiceal mucinous neoplasms are a heterogenous group of tumors that are typically classified into low- and high-grade histologic subtypes. The pathology of the peritoneal lesions associated with appendix cancers can be split into three groups: disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and PMCA with intermediate (well differentiated) features (17). Treatment of patients with disseminated mucinous neoplasms of the appendix has evolved over the last few decades. In 1987, Sugarbaker et al. reported the first use of IPC after CyRS in 14 patients with pseudomyxoma peritonei (18). In the ensuing three decades, there have been numerous studies showing promise in the use of IPC after CyRS for this type of tumor. A 2008 consensus article on the locoregional treatment of appendiceal mucinous neoplasms with peritoneal dissemination supported its basic role in managing this otherwise eventually fatal disease. The authors hoped that the rise of centers specialized in this method would help produce multi-center studies with less heterogenous populations, high-quality evidence and better post-surgical outcomes (19). However, there have been no RCTs completed to date evaluating the impact of cytoreduction and IPC for the treatment of appendix cancer. There are, however, multiple retrospective series reporting outcomes of therapy that we will include to cover the current landscape in the treatment of appendiceal cancer.
A 2012 observational study by Chua et al. reported superior progression free survival (PFS) associated with HIPEC after CyRS (HR, 0.65, P=0.03) for metastatic appendiceal mucinous neoplasm, but there was no OS difference in their multivariate analysis (20). The study included 2,298 patients who underwent HIPEC (60%), early postoperative intraperitoneal chemotherapy (EPIC) (2%) or a combination of the two (29%), with some patients undergoing neither treatment (9%). Postoperative complications occurred in 24% and post-surgical mortality was 2%. Median OS was 16.3 years, and median PFS was 8.2 years. The OS was strongly related to the completeness of cytoreduction (CCR). Patients who achieved a CCR0 (no macroscopic residual tumor disease) or CCR1 (residual tumor disease <2.5 mm) resection had significantly better OS (P<0.001) compared to those who underwent CCR2 (residual tumor disease 2.5 mm to 2.5 cm) or CCR3 (residual tumor disease >2.5 cm) resection, with the former group having 10-year OS rates in the 69-75% range as opposed to a dismal 10-year OS rate of 7% for the latter. The authors concluded that complete cytoreduction, rather than HIPEC, was the most important variable associated with improved survival. In contrast, an earlier retrospective Dutch study by Smeenk et al. in 2007 found pathologic subtype to be the most significant prognostic factor for disease-specific survival and disease-free survival (DFS) (21); however, CCR status became a significant factor once residual nodular disease was greater than 2.5 mm.
Another series of 64 patients with colon or appendiceal peritoneal metastases treated with CyRS followed by IPC at Memorial Sloan Kettering Cancer Center (MSK) from 1987 to 1999 also emphasized the importance of complete resection. The authors demonstrated that complete tumor resection was the only significant factor on multivariate analysis and was associated with a 54% 5-year survival versus a 16% 5-year survival for incomplete resection (22). A more recent report from MSK included 50 patients who underwent CyRS followed by EPIC for appendiceal mucinous neoplasm. The study demonstrated a 5-year recurrence free survival (RFS) of 43%. Seventeen patients experienced post-surgical complications and there were no perioperative mortalities. The 5-year DFS was 43% and median OS was 9.8 years (23). Another retrospective study in Norway of 93 patients compared EPIC and HIPEC following complete cytoreduction and showed no difference in 10-year OS and DFS (24).
Lymph node metastasis has been reported in as many as 40% of patients and is associated with decreased survival. Additionally, routine lymphadenectomy is not advocated in the setting of CyRS as it is unclear that it provides any benefit. However, some authors have demonstrated that long-term survival is still possible in this subset of patients after optimal CyRS/IPC is performed. Halabi et al., in a 2012 retrospective analysis of patients with appendiceal carcinomatosis, demonstrated an increase in 5-year OS rates of 21% in those with lymph nodes metastasis after optimal CyRS and HIPEC (25). This improvement in survival, however, has not been reproduced in the more recently published studies. Randle et al. reported the outcome of 31 patients with PC due to goblet cell appendiceal tumor pathology. A 36% complete cytoreduction rate was achieved and there was no significant difference in median OS in all patients, although patients with negative lymph nodes fared significantly better (median OS, 29.2 versus 10.2 months, P=0.002) than those with lymph nodes metastasis (26). Another study by Milovanov et al. divided 105 patients with PC of appendiceal origin according to whether they had undergone prior limited (LSG) or extensive surgery (ESG). All patients underwent CyRS/HIPEC after LSG or ESG. They found significantly worse OS among patients who had undergone ESG compared to those who underwent LSG (54% versus 26%, P=0.029), and this difference at 5 years was most significant in patients who had negative lymph nodes (17% versus 75%, P=0.026). There was no significant difference in outcome among those with positive lymph nodes who had prior ESG versus LSG, with both groups having a dismal 14% and 17% OS respectively at 5 years (P=0.61) (27). This study also underscored the importance of early referral to an experienced center rather than undergoing extensive surgical intervention prior to consideration for CyRS and HIPEC.
PFS and OS greater than 10 years have been frequently achieved for carcinomatosis of appendiceal origin, which is a significant improvement from historical controls treated with debulking and systemic therapy (28,29). This could be partially explained by the tendency of this type of tumor to stay within the peritoneal cavity. Decreased survival observed in patients who received systemic therapy is likely related to selection bias as those patients likely had more advanced or aggressive disease. Additionally, systemic metastasis is uncommon, occurring in 10% of cases (30,31). It is generally agreed upon now that the perioperative morbidity of CyRS/IPC can be justified by the potential long-term survival achieved for selected patients with peritoneal disease of appendiceal origin. As a result, many authors have frequently advocated CyRS with IPC to be the standard of care for this disease (19). Of note, there is an ongoing trial at MSK encompassing appendiceal cancers and the details of this trial will be discussed further below.
Colorectal cancer (CRC)
PC from CRC has been considered a terminal condition with dismal prognosis. As low as 10% 2-year OS with systemic chemotherapy and palliative surgery has been reported (32). Multiple phase II studies were completed in the 1990s to evaluate the role of CyRS and IPC for CRC with PC (33-35). The promising results from these studies encouraged other authors to evaluate the role of CyRS/IPC in randomized controlled settings. In 2003, the findings from a RCT comparing CyRS and IPC followed by systemic chemotherapy to systemic chemotherapy alone were reported. The study, which included 105 patients, demonstrated a median OS survival of 22.3 vs. 12.6 months in the systemic chemotherapy alone group. The perioperative mortality was significant (8%) after a median follow up of 21.6 months. OS was significantly associated with the CCR status (P<0.0001) and the initial peritoneal carcinomatosis index (PCI) score (36). Recently, the authors published longer term follow up (median 8-year) and showed that the 5-year DFS was 45% for patients with optimal cytoreduction and IPC compared to less than 10% for those with incomplete cytoreduction or systemic therapy arm alone. At the 6-year time point after treatment, only five and ten percent of patients in the standard and experimental arms, respectively, were alive. Although this study showed promising results, the findings were tempered by low participation rate, high mortality and a now outdated 5-FU/leucovorin regimen (37). Two other colorectal RCTs were opened in order to compare CyRS followed by EPIC (Europe) or HIPEC (USA) versus cytoreduction with systemic therapy. Unfortunately, however, both trials were closed due to failure to accrue patients (32,38).
In the void of additional trials, retrospective studies have shown superior outcome after CyRS, IPC, and systemic chemotherapy compared to standard chemotherapy with palliative surgery. Elias et al. compared 48 patients in both arms, and Franko et al. compared 67 patients with CyRS and HIPEC and 38 patients with standard therapy (39,40). Both studies demonstrated longer median OS (Elias et al. 62.7 vs. 23.9 months (P<0.05) and Franko et al. 34.7 vs. 16.8 months (P<0.001) in the treatment arms. The patients in both studies were highly selected with asymptomatic peritoneal disease and no extraperitoneal metastases. Modern systemic chemotherapy was used in both studies and showed improved survival rates compared with historic figures obtained from older chemotherapy regimens.
Comparing the two most common protocols for IPC delivery, Glehen et al. and Elias et al. reported their experience with both HIPEC and EPIC among patients with PC of CRC origin. The Glehen study analyzed the outcomes of 506 patients from 28 institutions worldwide who underwent HIPEC (54%), EPIC (24%) or both treatments (22%) with a median follow-up of 53 months. For CCR-0 patients, the associated 1-year, 3-year and 5-year survival rates were 87%, 47% and 31% in addition to a median survival time of 32.4 months. The OS rates were 72%, 39%, and 19% at 1, 3 and 5-year intervals respectively. The DFS trend was 40% at one year, 16% at three years, and 10% at the 5-year mark. Median OS was 19.2 months. There were 20 deaths post-operatively (4%) and 116 patients suffered major complications (22.9%) with a fistula being the most common complication (41). Six years later, Elias et al. analyzed 523 patients from 23 centers in France with a median follow-up of 45 months. Of the patients studied, the majority underwent HIPEC, 16% underwent EPIC and a fraction underwent both (1.7%) but no survival differences could be detected between HIPEC versus EPIC. Median OS was 30 months at five 5 years and there were 17 (3.3%) treatment-related deaths related to septic shock, respiratory embarrassment, hematologic toxicity, pulmonary embolism or acute renal insufficiency (42). Overall 1-, 3- and 5-year survival rates were 81%, 41% and 27% and the DFS rates were quite similar to the Glehen paper. Grade 3 and 4 complications occurred in 31% with reoperation, fistula, hemorrhage, abscess and lung infection as the most common. In the 416 patients who underwent complete CyRS, multivariate analysis identified extent of carcinomatosis, presence of liver metastases, center experience, lymph node status and adjuvant chemotherapy as associated with clinical outcome. These studies showed that mortality was low (3-4%) and morbidity rates were acceptable (20-30%) which supported this approach as a viable treatment option especially when complete CyRS can be performed.
Finally, Prodige 7, a highly anticipated, French multi-centric trial, has completed inclusion of 280 patients as planned. This study seeks to quantify the impact of HIPEC, as it compares patients randomized to HIPEC or not after CyRS. Patients in this trial had a PCI below 25. There are no definitive results of this trial available yet (https://www.clinicaltrialsregister.eu/ctr-search/search?query=PRODIGE+7), but early work from the same group of authors indicates that that the median survival is higher than expected in both groups (43).
Advances in systemic chemotherapy over the last two decades have resulted in improving survival rates in metastatic CRC. However, those patients with PC still have a poorer outcome compared to those with other sites of metastasis. CyRS followed by IPC for isolated peritoneal metastasis is routinely combined with systemic therapy. Additionally, some studies have demonstrated that patients with extensive peritoneal disease burden do poorly with cytoreduction and IPC and, therefore, should be selectively treated with this aggressive approach (42,44). Laparoscopy or magnetic resonance imaging prior to laparotomy can be valuable in patients with equivocal evidence of extensive peritoneal disease. CyRS and IPC is associated with better than expected survival in selected patients with peritoneal metastasis from CRC. However, the optimal protocol for IPC administration and the role for CyRS and IPC in patients with resectable liver metastases is not yet known.
Ongoing trials worldwide for colon, rectal and appendiceal cancers
A randomized, non-blinded, phase II clinical trial is currently ongoing at MSK. This trial is entitled (Intraperitoneal Chemotherapy After cytoReductive Surgery). It compares post-operative Intraperitoneal Chemotherapy (EPIC) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) After Optimal Cytoreductive Surgery (CyRS) for Neoplasms of the Appendix, Colon or Rectum With Isolated Peritoneal Metastasis’ (https://clinicaltrials.gov/ct2/show/NCT01815359). This is the first randomized trial comparing early EPIC or HIPEC for appendiceal and CRC. The purpose of this study is to measure the efficacy and toxicity of EPIC and HIPEC after CyRS among patients with appendiceal, rectal or colon cancer and isolated PC. The primary outcome measure is 3-year DFS. Documentation of tumor recurrence will be made based on surveillance CT scans with clinical correlation from the treating physician. The secondary outcome measures are grades 3 to 5 morbidity at 60 days. The accrual target is 212 patients. As of May 2015, 63 patients have been randomized and the trial is on track to be completed by 2019. Patients will be stratified by previous systemic chemotherapy and by the organ of origin. Patients are randomized to either HIPEC (group A) or EPIC (group B) after the operating surgeon determines that the patient can undergo an optimal cytoreduction. The ICARuS trial stratification and interventions are outlined in Figure 1. Additional important trials are ongoing worldwide and are listed in Table 1 in order of estimated year of completion as reviewed on www.clinicaltrials.gov.
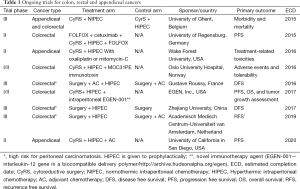
Full table
Gastric cancer
PC of gastric origin has traditionally been considered incurable, with a dire prognosis. A recent prospectively collected study of 1,108 patients with gastric adenocarcinoma who then subsequently developed a metachronous PC revealed a median survival of 3 months despite an initial R0 D2 resection in those with metachronous peritoneal recurrence (45). Prior studies have demonstrated similar median survival of up to 6 months and as short as 3 months (46). Conventionally, the standard treatment for gastric cancer with PC has been systemic chemotherapy, which has improved median survival to 7-10 months (47). However, in patients with peritoneal metastases from gastric cancer, even systemic chemotherapy has low response rates (48). In 1988, Fujimoto et al., reported the first series of IPC in 15 patients with PC from gastric cancer with a mean survival rate of 7.2±4.6 months (49). Additionally, the addition of intraoperative saline lavage (extensive intraoperative peritoneal lavage) followed by IPC with cisplatin by a Japanese group showed significant improvement in five-year survival in 88 patients with gastric cancer (50).
A subsequent study of 159 patients who received CyRS followed by IPC (HIPEC in 150 and EPIC in the rest) demonstrated a median survival of 9.2 months and 5-year survival rates of up to 13% (51). Older reports of Japanese studies reported no improvement in survival with IPC in 21 patients; in fact, the authors reported increased respiratory and renal failures (52), which had led to conflicting information and hesitation regarding the use of IPC in gastric cancer with peritoneal tumor burden (53). In 2011, Yang et al. reported results of a RCT comparing CyRS and IPC against CyRS alone among 68 patients with isolated peritoneal metastasis from gastric cancer. Median OS was 11.0 months in the CyRS/IPC group and 6.5 months in the CyRS alone group (P<0.05) (54).
Thus, the gastrectomy with CyRS (metastasectomy) plus systemic chemotherapy (GYMS) versus systemic chemotherapy alone (SA) study was an attempt to reconcile these conflicting studies (also known as GYMSSA). This study compared gastrectomy with CyRS and HIPEC plus systemic therapy with systemic therapy alone. The study was designed to compare the two therapeutic approaches, unfortunately, it was far too small to be definitive; seven patients received CyRS, HIPEC, and systemic FOLFOXIRI chemotherapy as opposed to nine patients who received only FOLFOXIRI. Of the patients who received chemotherapy alone, none survived longer than 11 months; by contrast, the patient in the cytoreductive group with HIPEC and FOLFOXIRI survived as long as two years. Median OS was 4.3 months (chemotherapy alone) versus 11.3 months (CyRS, HIPEC and systemic chemotherapy) (55). These results lent more optimism to the use of HIPEC, and led to further trials solidifying the efficacy of HIPEC as an adjunctive treatment for gastric cancer.
One of the interesting questions raised recently by Wu et al., is regarding the timing of CyRS and IPC. They found that staged CyRS and IPC yielded better OS and less morbidity than patients who had simultaneous CyRS (56). Since the Yang trial demonstrated CyRS plus IPC was associated with increased OS, and the GYMSSA trial sought to clarify this difference even in the face of systemic chemotherapy, additional randomized trials are now ongoing and their results are eagerly awaited. There are three currently active gastric cancer trials in France, Spain, and Texas (Table 2, listed in order of estimated completion) as reviewed on www.clinicaltrials.gov.
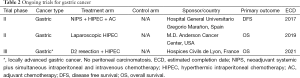
Full table
Diffuse malignant peritoneal mesothelioma
Peritoneal mesothelioma is a rare malignancy; in the US, its annual incidence approximates 100-400 cases (57,58). The origin of this tumor is believed to be the mesothelium of the abdominal cavity, which progress to a nodule and then to plaque formation with aggressive locoregional invasion and mass effect. There are several variants of malignant mesothelioma, but diffuse malignant peritoneal mesothelioma comprises only 10% of mesothelioma at large (59). Diffuse malignant peritoneal mesothelioma itself can be described histologically as either epithelioid, sarcomatoid, or biphasic; with the epithelioid type associated with the best prognosis (60). Peritoneal mesothelioma is commonly treated with systemic chemotherapy (pemetrexed, cisplatin and gemcitabine), and occasionally palliative surgery and total abdominal radiation (61). Of note, systemic chemotherapy with cisplatin and pemetrexed is the only FDA-approved systemic regimen for this entity (62). Median survival with pemetrexed and gemcitabine or cisplatin ranges from 12 to 26.8 months (63,64).
A number of trials in development for peritoneal mesothelioma are in process, but no current trials are available to report. CyRS is thought to be efficacious in this disease by reducing recurrence in peritoneal mesothelioma; 86% of recurrences take place in the peritoneal cavity, with incomplete cytoreduction being an independent predictor of intraperitoneal recurrence (65,66). Brigand et al. reviewed fifteen patients with peritoneal mesothelioma who had received CyRS and HIPEC between 1989 and 2004; median survival was 35.6 months overall and the authors concluded that the only significant factors affected median survival were CCR and extent of carcinomatosis (67). Additionally, Baratti et al. demonstrated, retrospectively, that patients receiving a complete parietal peritonectomy had increased 5-year survival compared to their cohorts who had only received a selective parietal peritonectomy without any difference in morbidity (68).
A prospective study of twelve patients treated with HIPEC combined with CyRS reported median survival of 34.2 months and complete resolution of malignant ascites in 86% of patients (69). A similar report of forty-nine patients reported a median OS of 92 months, while another series of twenty-six patients with a different HIPEC regimen at a different institution reported median survival of 100 months with a 5-year survival rate of 63%, but at the cost of 54% perioperative morbidity (70,71). One of the largest series to date has studied 401 patients (complete follow-up) who received CyRS and HIPEC; mean survival was 53 months (72); a smaller series of seventeen patients reported a median survival of 3.7 years (73). Epithelial subtype, absence of lymph node metastases, complete cytoreduction (CC-0 or CC-1) and HIPEC were all associated with improved survival in the series of 401 patients (P<0.05). Although a variety of chemotherapy regimens have been used between the studies mentioned above, Blackham et al. studied thirty-four patients with peritoneal mesothelioma receiving either mitomycin or cisplatin and concluded that median OS and PFS were both higher in the cisplatin group; therefore the authors concluded by recommending the use of cisplatin for HIPEC (74).
Moreover, the timing of systemic chemotherapy in the setting of CyRS and IPC is not clear. One retrospective study did not show any associated differences in median survival or morbidity between groups who received systemic chemotherapy (pre- or post-operatively) and those who did not (75). The choice of IPC approach varies; Shetty et al. reported better outcomes and fewer complications in patients who underwent CyRS and HIPEC with carboplatin when compared to those receiving HIPEC with mitomycin (76). Recently, a meta-analysis of 20 retrospective studies reporting on 1,047 patients with malignant peritoneal mesothelioma was published. Pooled estimates of survival yielded a 1-, 3- and 5-year survival of 84%, 59%, and 42%, respectively. Patients received EPIC and those who received cisplatin-based IPC had an associated improved 5-year survival. The median PCI score was 19. Complete cytoreduction (CCR 0&1) was performed in 67% (range: 46-93%) of patients (77).
Newer studies currently underway are studying the safety and efficacy of IPC in patients with phase II trials for peritoneal mesothelioma. Interestingly, since recurrence occurs frequently in this malignancy, and is the usual culprit of eventual mortality, the latest research suggests that iterative CyRS and IPC may improve outcomes regarding morbidity and symptomatology, though they have demonstrated better outcomes at median time points (78). Clearly the state of modern treatment in Diffuse Malignant Peritoneal Mesothelioma is in evolution and more studies regarding reiterative CyRS and IPC are needed to confirm this association.
Ovarian cancer (OC)
OC is the sixth most common malignancy in women worldwide and the most deadly gynecological malignancy (79,80). About 70% of patients with OC are diagnosed with advanced disease at either stage 3 or 4 (81). Classically, OC has been treated with debulking surgery and adjuvant chemotherapy. The median 5-year survival is less than 50% and up to 70% of all OC patients with relapse eventually die of this disease (82,83). OC also has the tendency to spread throughout the peritoneal cavity. Alberts et al. reported the first landmark study comparing systemic chemotherapy and IPC, in the delayed post-operative setting, following the resection of all peritoneal masses greater than 2 cm (84). Patients who received post-operative IPC had a median survival of 49 months compared to 41 months for the systemic chemotherapy group.
Consistent with the previously discussed malignancies, outcomes are strongly related to the CCR. With optimal debulking, defined as residual disease less than 1 cm diameter, median OS ranging from 49 to 66 months has been reported (85,86). The Gynecologic Oncology Group compared systemic cisplatin and paclitaxel to systemic carboplatin and paclitaxel combined with post-operative IP cisplatin after an optimal cytoreduction to less than 1 cm in both groups. Patients who received IPC had an associated improvement of PFS (28 versus 22 months) and OS (63 versus 52 months) (85). Similarly, Armstrong et al. reported an associated improvement in PFS (23.8 versus 18.3 months) and OS (65.6 vs. 49.7 months) in the systemic and IPC group compared to the systemic chemotherapy group after complete cytoreduction in both groups (86). Recent work from Chi et al. at MSK supports the notion that advanced stage OC with bulky stage IIIC-IV disease should be treated with primary debulking surgery in most cases and the use of neoadjuvant chemotherapy should be reserved for patients where complete cytoreduction cannot be done or in patients unable to tolerate CyRS (87).
A multi-institutional phase II study was recently completed evaluating PFS and OS in 26 women with stage 3 and 4 OC treated with CyRS and HIPEC. All patients underwent CyRS, followed by HIPEC with cisplatin and doxorubicin. Subsequently, patients received adjuvant systemic carboplatin and paclitaxel. Optimal cytoreduction was achieved in 57% of patients and 43% had minimal residual disease (less than 2.5 mm). OS was 60.7% and PFS was 15.2% at 5 years (median, 30 months) (88). One of the largest and most recent studies is a retrospective, multi-centric (13 centers) French cohort that analyzed the data of 566 patients with advanced or recurrent OC [446]. They achieved a CCR0 rate of 74.9% and perioperative morbidity and mortality was at 31.3% and 0.8% respectively. Median OS was at 35.4 and 45.7 months for advanced and recurrent OC. This analysis determined PCI, not CCR, as associated with the best prediction of survival (89).
A Greek phase III randomized prospective trial has also been recently published. The authors randomized 120 patients with stage IIIc or IV OC that had recurred after debulking and systemic chemotherapy to CyRS with HIPEC vs. CyRS without HIPEC. Both arms received systemic chemotherapy. They found a significant increase in mean survival in the HIPEC group (26.7 versus 13.4 months, P<0.006) with 3-year survival at 75% vs. 18% (P<0.01). Even more interesting, the differences in survival between those with and without platinum resistance was not significant in the HIPEC group (26.6 versus 26.8 months, P<0.3) as it was for the systemic chemotherapy group (15.2 versus 10.2 months, P<0.002). The HIPEC group had an approximately 10% increased proportion of stage IIIc patients (68%) in comparison to the non-HIPEC group (58%) (90). Nonetheless, it further emphasizes the promise of HIPEC and the need for further studies.
Studies concerning IPC in OC are difficult to interpret due to the lack of a universal definition of optimal cytoreduction (less than 2 cm, less than 1 cm or less than 2.5 mm), the variety of agents used for IPC and the optimal timing of IPC in the disease process (frontline, for recurrence or for consolidation). According to the National Comprehensive Cancer Network (NCCN) guidelines, post-operative IPC remains the standard of care for patients with PC due to OC; HIPEC remains a promising but yet unproven therapy in this setting.
Ongoing trials worldwide for ovarian, fallopian and peritoneal Cancers
A phase II randomized trial for patients with ovarian, fallopian or peritoneal cancers is led by Dr. Dennis Chi at Memorial Sloan Kettering in collaboration with Holy Cross Hospital in Maryland, Hartford Healthcare and the Mayo Clinic. It is referred to as a ‘Phase II Study of Secondary Cytoreduction with and without HIPEC plus Postoperative Chemotherapy for Women with Recurrent Ovarian, Fallopian Tube, or Primary Peritoneal Cancer’ (https://clinicaltrials.gov/ct2/show/NCT01767675).
The purpose of this study is to examine if CyRS and HIPEC, followed by standard postoperative chemotherapy, is more effective than standard postoperative chemotherapy without HIPEC for women with recurrent ovarian, fallopian tube, or primary peritoneal cancer who are undergoing a second surgery to remove peritoneal disease. Patients will be randomized intraoperatively to undergo CyRS with HIPEC (arm A) or CyRS only (arm B) in a 1:1 manner (see Figure 2 for the schema). Patients in both arms will receive a standard platinum-based systemic chemotherapy postoperatively (5 cycles in arm A and 6 cycles in arm B). In some patients randomized to HIPEC at MSK only, peritoneal fluid and blood samples will be drawn before, during and after the HIPEC procedure. The primary outcome measure is to determine the proportion of patients who are without evidence of disease progression at 24 months. A proportion of patients of ≥40%, who are without evidence of disease progression at 24 months, is considered acceptable, whereas a proportion of ≤25% is considered unacceptable in this patient population.
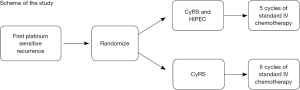
The secondary outcome measures are the following: (I) to determine the toxicity and postoperative complication rates at 4 weeks post-operatively. The safety endpoint of this trial is to determine toxicity and postoperative complication rates in both arms using NCI Common Terminology Criteria for Adverse Events version 4.0; (II) to determine the completion rate of four cycles at 5 years; (III) to complete a secondary analysis estimating the completion rate. The completion rate and a 95% confidence interval will be calculated for each arm separately. Completion is defined as patients being able to complete ≥4 out of 5 or 6 cycles of a standard systemic chemotherapy; (IV) to characterize the pharmacokinetics within 5 years in a subset of patients randomized to receive HIPEC in the OR; (V) to evaluate peritoneal fluid and blood samples in patients randomized to HIPEC from MSK patients for potential biomarkers. The estimated accrual is 98 patients and 21 patients (10 HIPEC, 11 non-HIPEC) are currently on study. The estimated primary date of completion is June 2018. Ongoing trials worldwide evaluating CyRS and IPC in ovarian, fallopian and peritoneal cancers can be found in Table 3 in order of estimated completion as reviewed on www.clinicaltrials.gov.

Full table
Trials for mixed types of peritoneal cancer
A number of trials worldwide are open to a broad array of peritoneal cancer types to augment numbers and to expeditiously answer the fundamental issues of efficacy and safety of IPC and cytoreduction. Given the anticipated difficulty in achieving adequate power with low accrual in rare disease types, these trials seek to reduce the time it takes to complete a trial by incorporating patient groups representing multiple tumor types. The limitation of this particular trial design could be dilution of any particular effect that could pertain to an individual tumor type and resultant inability to support the use of IPC. Ongoing trials for mixed types of cancer are listed in Table 4 in order of estimated completion date as reviewed on www.clinicaltrials.gov.
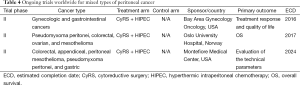
Full table
Conclusions
CyRS and IPC are now being used as viable treatment options in highly selected patients with metastatic spread to the peritoneal cavity. Outcomes of therapy vary with primary disease histology. The survival advantage varies from a few years in the case of PC of appendiceal origin compared to historical controls, in contrast to only a few months for patients with gastric cancer associated peritoneal carcinomatoses. The data clearly show that CyRS is associated with better outcomes when a complete cytoreduction is achieved whereas incomplete cytoreduction is associated with poor survival. Considering the significant cost and morbidity of CyRS and IPC, proper patient selection cannot be overemphasized. CyRS and IPC can result in long-term survival and possibly cure for PC from colorectal, appendiceal, primary peritoneal, and OCs. However, no data exist supporting the notion that CyRS and IPC can achieve long-term survival for other tumor histologies such as pancreatic and primary hepatic as well breast, sarcoma and melanoma. Currently there is no standard protocol for IPC and the efficacy of EPIC and HIPEC are being evaluated in multiple ongoing RCTs, and we eagerly look forward to the results of these studies. The two trials currently ongoing at MSK are examples of modern, randomized phase II trials that will provide much needed prospective data to temper the available literature and better guide appropriate treatment regimens. These trials along with many others will provide the basis for a more standardized and evidence-based approach to the treatment of patients with colorectal, appendiceal or ovarian PC, which will be important to optimize outcomes for patients with these challenging disease processes.
Acknowledgements
The authors would like to thank Dr. Dennis Chi and his team for gracious use of information and material for Figure 2.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jafari MD, Halabi WJ, Stamos MJ, et al. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the american college of surgeons national surgical quality improvement program. JAMA Surg 2014;149:170-5. [PubMed]
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [PubMed]
- Ung L, Chua T, Morris D. Peritoneal metastases of lower gastrointestinal tract origin: A comparative study of patient outcomes following cytoreduction and intraperitoneal chemotherapy. J Cancer Res Clin Oncol 2013;139:1899-908. [PubMed]
- Brücher BL, Piso P, Verwaal V, et al. Peritoneal Carcinomatosis: Cytoreductive Surgery and HIPEC-Overview and Basics. Cancer Invest 2012;30:209-24. [PubMed]
- Kelly KJ, Nash GM. Peritoneal debulking/intraperitoneal chemotherapy-non-sarcoma. J Surg Oncol 2014;109:14-22. [PubMed]
- Sugarbaker PH, Schellinx ME, Chang D, et al. Peritoneal carcinomatosis from adenocarcinoma of the colon. World J Surg 1996;20:585-591; discussion 592. [PubMed]
- Sugarbaker PH. Peritonectomy procedure. Ann Surg 1995;221:29-42. [PubMed]
- Rufián S, Muñoz-Casares FC, Briceño J, et al. Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol 2006;94:316-24. [PubMed]
- Hayes-Jordan A, Green H, Prieto V, et al. Unusual cases: Melanomatosis and nephroblastomatosis treated with hyperthermic intraperitoneal chemotherapy. J Pediatr Surg 2012;47:782-7. [PubMed]
- Cardi M, Sammartino P, Framarino ML, et al. Treatment of peritoneal carcinomatosis from breast cancer by maximal cytoreduction and HIPEC: A preliminary report on 5 cases. Breast 2013;22:845-9. [PubMed]
- Honore C, Goere D, Dartigues P, et al. Peritoneal carcinomatosis from solid pseudopapillary neoplasm (Frantz’s tumour) of the pancreas treated with HIPEC. Anticancer Res 2012;32:1069-73. [PubMed]
- Tabrizian P, Franssen B, Jibara G, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with peritoneal hepatocellular carcinoma. J Surg Oncol 2014;110:786-90. [PubMed]
- Elias D, David A, Sourrouille I, et al. Neuroendocrine carcinomas: Optimal surgery of peritoneal metastases (and associated intra-abdominal metastases). Surgery 2014;155:5-12. [PubMed]
- Munene G, Mack LA, Temple WJ. Systematic review on the efficacy of multimodal treatment of sarcomatosis with cytoreduction and intraperitoneal chemotherapy. Ann Surg Oncol 2011;18:207-13. [PubMed]
- Baratti D, Pennacchioli E, Kusamura S, et al. Peritoneal sarcomatosis: is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Ann Surg Oncol 2010;17:3220-8. [PubMed]
- Kallianpur AA, Shukla NK, Deo SV, et al. Updates on the multimodality management of desmoplastic small round cell tumor. J Surg Oncol 2012;105:617-21. [PubMed]
- Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 1995;19:1390-408. [PubMed]
- Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma peritonei of colonic origin. Natural history and presentation of a curative approach to treatment. Dis Colon Rectum 1987;30:772-9. [PubMed]
- Moran B, Baratti D, Yan TD, et al. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol 2008;98:277-82. [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [PubMed]
- Smeenk RM, Verwaal VJ, Antonini N, et al. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg 2007;245:104-9. [PubMed]
- Culliford AT, Brooks AD, Sharma S, et al. Surgical debulking and intraperitoneal chemotherapy for established peritoneal metastases from colon and appendix cancer. Ann Surg Oncol 2001;8:787-95. [PubMed]
- Wagner PL, Jones D, Aronova A, et al. Early Postoperative Intraperitoneal Chemotherapy Following Cytoreductive Surgery for Appendiceal Mucinous Neoplasms With Isolated Peritoneal Metastasis. Dis Colon Rectum 2012;55:407-15. [PubMed]
- Sørensen O, Flatmark K, Reed W, et al. Evaluation of complete cytoreductive surgery and two intraperitoneal chemotherapy techniques in pseudomyxoma peritonei. Eur J Surg Oncol 2012;38:969-76. [PubMed]
- Halabi HE, Gushchin V, Francis J, et al. Prognostic Significance of Lymph Node Metastases in Patients with High-Grade Appendiceal Cancer. Ann Surg Oncol 2012;19:122-5. [PubMed]
- Randle RW, Griffith KF, Fino NF, et al. Appendiceal goblet cell carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Res 2015;196:229-34. [PubMed]
- Milovanov V, Sardi A, Aydin N, et al. Extensive surgical history prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is associated with poor survival outcomes in patients with peritoneal mucinous carcinomatosis of appendiceal origin. Eur J Surg Oncol 2015;41:881-5. [PubMed]
- Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg 1994;219:112-9. [PubMed]
- Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 2005;241:300-8. [PubMed]
- Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol 2007;14:2289-99. [PubMed]
- Zoetmulder FA, Sugarbaker PH. Patterns of failure following treatment of pseudomyxoma peritonei of appendiceal origin. Eur J Cancer 1996;32A:1727-33. [PubMed]
- Elias D, Delperro JR, Sideris L, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol 2004;11:518-21. [PubMed]
- Cavaliere F, Perri P, Di Filippo F, et al. Treatment of peritoneal carcinomatosis with intent to cure. J Surg Oncol 2000;74:41-4. [PubMed]
- Fujimura T, Yonemura Y, Fujita H, et al. Chemohyperthermic peritoneal perfusion for peritoneal dissemination in various intra-abdominal malignancies. Int Surg 1999;84:60-6. [PubMed]
- Schneebaum S, Arnold MW, Staubus A, et al. Intraperitoneal hyperthermic perfusion with mitomycin C for colorectal cancer with peritoneal metastases. Ann Surg Oncol 1996;3:44-50. [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [PubMed]
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32. [PubMed]
- Klaver YL, Simkens LH, Lemmens VE, et al. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol 2012;38:617-23. [PubMed]
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-5. [PubMed]
- Franko J, Ibrahim Z, Gusani NJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010;116:3756-62. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [PubMed]
- Elias D, Goéré D, Dumont F, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer 2014;50:332-40. [PubMed]
- Hompes D, D’Hoore A, Van Cutsem E, et al. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol 2012;19:2186-94. [PubMed]
- Seyfried F, von Rahden BH, Miras AD, et al. Incidence, time course and independent risk factors for metachronous peritoneal carcinomatosis of gastric origin--a longitudinal experience from a prospectively collected database of 1108 patients. BMC Cancer 2015;15:73. [PubMed]
- Shen P, Stewart JH, Levine EA. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancy: overview and rationale. Curr Probl Cancer 2009;33:125-41. [PubMed]
- Pyrhönen S, Kuitunen T, Nyandoto P, et al. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587-91. [PubMed]
- Yonemura Y, Endou Y, Sasaki T, et al. Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur J Surg Oncol 2010;36:1131-8. [PubMed]
- Fujimoto S, Shrestha RD, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg 1988;208:36-41. [PubMed]
- Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009;250:242-6. [PubMed]
- Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370-7. [PubMed]
- Kunisaki C, Shimada H, Akiyama H, et al. Therapeutic outcomes of continuous hyperthermic peritoneal perfusion against advanced gastric cancer with peritoneal carcinomatosis. Hepatogastroenterology 2006;53:473-8. [PubMed]
- Königsrainer I, Horvath P, Struller F, et al. Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J Gastric Cancer 2014;14:117-22. [PubMed]
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [PubMed]
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. [PubMed]
- Wu X, Li Z, Li Z, et al. Hyperthermic intraperitoneal chemotherapy plus simultaneous versus staged cytoreductive surgery for gastric cancer with occult peritoneal metastasis. J Surg Oncol 2015;111:840-7. [PubMed]
- Teta MJ, Mink PJ, Lau E, et al. US mesothelioma patterns 1973-2002: indicators of change and insights into background rates. Eur J Cancer Prev 2008;17:525-34. [PubMed]
- SEER Cancer Statistics Review-Mesothelioma fast stats. Available online: http://seer.cancer.gov/faststats/selections.php?series=cancer
- Attanoos RL, Gibbs AR. Pathology of malignant mesothelioma. Histopathology 1997;30:403-18. [PubMed]
- Yan TD, Welch L, Black D, et al. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 2007;18:827-34. [PubMed]
- Ahmed S, Stewart JH, Shen P, et al. Outcomes with cytoreductive surgery and HIPEC for peritoneal metastasis. J Surg Oncol 2014;110:575-84. [PubMed]
- Scagliotti GV, Selvaggi G. Emerging drugs for mesothelioma. Expert Opin Emerg Drugs 2007;12:127-37. [PubMed]
- Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211-8. [PubMed]
- Simon GR, Verschraegen CF, Jänne PA, et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol 2008;26:3567-72. [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Diffuse malignant peritoneal mesothelioma: Failure analysis following cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2009;16:463-72. [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Ann Surg Oncol 2012;19:1416-24. [PubMed]
- Brigand C, Monneuse O, Mohamed F, et al. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol 2006;13:405-12. [PubMed]
- Baratti D, Vaira M, Kusamura S, et al. Multicystic peritoneal mesothelioma: outcomes and patho-biological features in a multi-institutional series treated by cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Eur J Surg Oncol 2010;36:1047-53. [PubMed]
- Loggie BW, Fleming RA, McQuellon RP, et al. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg 2001;67:999-1003. [PubMed]
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7. [PubMed]
- Elias D, Bedard V, Bouzid T, et al. Malignant peritoneal mesothelioma: treatment with maximal cytoreductive surgery plus intraperitoneal chemotherapy. Gastroenterol Clin Biol 2007;31:784-8. [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [PubMed]
- Yano H, Moran BJ, Cecil TD, et al. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal mesothelioma. Eur J Surg Oncol 2009;35:980-5. [PubMed]
- Blackham AU, Shen P, Stewart JH, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: mitomycin versus cisplatin. Ann Surg Oncol 2010;17:2720-7. [PubMed]
- Deraco M, Baratti D, Hutanu I, et al. The role of perioperative systemic chemotherapy in diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2013;20:1093-100. [PubMed]
- Shetty SJ, Bathla L, Govindarajan V, et al. Comparison of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy with mitomycin or carboplatin for diffuse malignant peritoneal mesothelioma. Am Surg 2014;80:348-52. [PubMed]
- Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:1686-93. [PubMed]
- Ihemelandu C, Bijelic L, Sugarbaker PH. Iterative cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent or progressive diffuse malignant peritoneal mesothelioma: clinicopathologic characteristics and survival outcome. Ann Surg Oncol 2015;22:1680-5. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Coleman RL, Monk BJ, Sood AK, et al. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol 2013;10:211-24. [PubMed]
- Horner MJ, Ries LA, Kracho M et al. SEER Cancer Statistics Review, 1975-2006. Bethesda, MD. 2009. Available online: http://seer.cancer.gov/archive/csr/1975_2006/
- Teo MC. Update on the management and the role of intraperitoneal chemotherapy for ovarian cancer. Curr Opin Obstet Gynecol 2014;26:3-8. [PubMed]
- Alberts DS, Liu PY, Hannigan E V, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950-5. [PubMed]
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecol. J Clin Oncol 2001;19:1001-7. [PubMed]
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34-43. [PubMed]
- Chi DS, Musa F, Dao F, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol 2012;124:10-4. [PubMed]
- Deraco M, Kusamura S, Virzì S, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: multi-institutional phase-II trial. Gynecol Oncol 2011;122:215-20. [PubMed]
- Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 2013;39:1435-43. [PubMed]
- Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 2015;22:1570-5. [PubMed]



