
Construction of a survival prediction model for patients with hepatocellular carcinoma (HCC) based on real clinical data: a single-center retrospective study
Highlight box
Key findings
• We constructed a novel nomogram for predicting survival outcomes in patients with hepatocellular carcinoma (HCC).
What is known and what is new?
• The overall survival prognosis of HCC is poor, and accurate survival prediction tools are needed in the treatment.
• We present a data-driven nomogram based on a large real-world clinical dataset. It identifies patients without HCC screening, tumor size >5 cm, alcoholic liver disease, Child-Pugh grade C, and those with conservative treatment as having poor prognosis.
What is the implication, and what should change now?
• Enhances risk stratification and personalized treatment.
• Integrate into clinical practice; expand HCC screening; validate externally.
Introduction
Primary liver cancer is one of the most common malignant tumors and a leading cause of mortality in patients with chronic liver disease worldwide. Globally, it ranks second in cancer-related fatalities and is the seventh most prevalent type of cancer (1). The highest incidences are found in Asia and Africa, with Mongolia having the highest prevalence at 93.7 cases per 100,000 population (1). However, due to its incidence rate of 18.3 cases per 100,000 population, and the largest population in the world (1.4 billion), China has the highest number of cases (2).
Since primary liver cancer has a dismal prognosis worldwide, rates of morbidity and death are nearly equal. Primary liver cancer was expected to have an incidence of 9.3 cases per 100,000 person-years worldwide in 2018, with a corresponding mortality rate of 8.5 deaths per 100,000 person-years (3). The World Health Organization (WHO)’s International Agency for Research on Cancer (IARC) has predicted that by 2040, new cases and fatalities from primary liver cancer might rise by 55% globally (4).
Moreover, primary liver cancer is currently the fourth most prevalent malignant tumor in China. Furthermore, it is the second leading cause of tumor-related deaths within China, posing a major risk to the lives and health of Chinese citizens (5). The majority of liver cancer diagnoses and fatalities are attributed to hepatocellular carcinoma (HCC), the predominant histological form of liver cancer. In this study, HCC is used as the abbreviation for primary liver carcinoma (6).
The primary influencing variables for individuals with HCC have been documented in several studies; however, further information is required to validate the independent risk variables for prognosis and survival (7,8). As a result, we retrospectively gathered and examined pertinent information from patients’ medical records who had HCC for this research. The demographic and clinicopathological features of those suffering from HCC were analyzed to construct a nomogram model and its predictive value was determined to offer a foundation for the clinical management of patients and afterward follow-up prognosis of HCC. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-806/rc).
Methods
Study patients
A total of 1,128 individuals with primary HCC who were discharged from The First Affiliated Hospital of Zhengzhou University between January 1, 2011, and December 31, 2019, participated in the present investigation. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Zhengzhou University (No. ZZUIRB2022-151) and individual consent for this retrospective analysis was waived.
Inclusion criteria
The study participants had to meet the following requirements to be eligible: (I) final diagnosis of HCC; (II) age at diagnosis of HCC ≥18 years; (III) no prior therapy received in other facilities before HCC diagnosis; (IV) no complications related to human immunodeficiency virus (HIV), syphilis, leukemia, or other serious immune system diseases; (V) absence of any other cancerous tumors; and (VI) availability of comprehensive clinical and post-treatment data.
Exclusion criteria
The following were the study’s exclusion criteria: (I) HCC was not diagnosed; (II) the patient was diagnosed with intrahepatic cholangiocarcinoma (ICC) or mixed ICC with HCC (HCC-ICC); (III) liver cancer before January 1, 2011; (IV) additional severe immune system conditions; or (V) excluding patients with malignancies other than HCC; and (VI) unstageable clinical or follow-up data were lacking.
Data collection
The primary information gathered for this real-world research included patient demographics, imaging and serological results, treatment schedules, and traceable prognoses. The patient demographic and sociological characteristics collected were: (I) length of hospital stay; (II) sex; (III) age; (IV) dates of discharge and admittance; (V) clinical condition upon release; (VI) the existence of long-term illnesses (e.g., hypertension, diabetes, and stroke); (VII) family history of cancer; (VIII) the presence of chronic viral hepatitis; (IX) the existence of liver cirrhosis; and (X) whether the patient had received liver cancer screening.
Reviewing patient diagnostic imaging data included liver biopsy records, intraoperative pictures taken at the time of HCC diagnosis, and results from computed tomography (CT) and magnetic resonance imaging (MRI). Among the information gathered were the following: (I) tumor count, (II) maximal diameter of a single tumor, (III) extrahepatic or lymph node metastasis, (IV) level of tumor differentiation, (V) presence and severity of ascites, and (VI) existence of portal hypertension. The patient serological reports were: (I) alpha-fetoprotein (AFP), (II) total bilirubin, (III) albumin levels, and (IV) prothrombin time.
Follow-up
At both the hospital and the outpatient clinic, patients received routine follow-up care. Patients without clinical results in the hospital were routinely phoned by their relatives to inquire about their survival status. Each patient was followed up to 5 years following surgery or until an outcome event occurred, with the follow-up deadline set as December 31, 2020.
Statistical analysis
First, the t-test was employed to compare categories, and the normally distributed measurement results were expressed as means ± standard deviation (). The medians (P25, P75) were utilized to characterize non-normally distributed measurement information, and non-parametric analyses were employed to compare categories. Count information was defined as the total amount of cases and proportions. The Chi-squared (χ2) test or Fisher’s exact probability was employed to evaluate group comparisons. Second, we eliminated research factors unrelated to overall survival (OS) (P>0.05) using a univariate Cox regression study and Kaplan-Meier survival.
The independent risk variables for OS were discovered employing multivariate Cox regression analysis (P<0.05). The 1-, 3-, and 5-year OS nomogram prediction models for patients with HCC were created according to independent risk variables. Receiver operating characteristic (ROC), calibration, and decision curve analysis [DCA; which quantifies a model’s clinical utility by comparing its net benefit across threshold probabilities to reference strategies, where clinical usefulness is defined as the DCA curve lying above both the “treat all” and “treat none” lines (9)] were used to evaluate the nomogram’s prognostic accuracy and clinical relevance. All statistical analyses were completed employing the SPSS (version 21.0) and R (version 4.1.3) programs. A P value <0.05 (two-sided) was considered statistically significant.
Results
Demographic and baseline features of patients
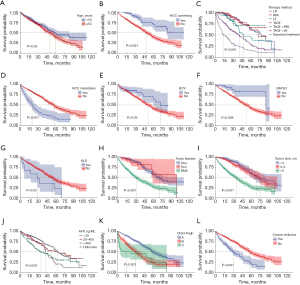
We enrolled 1,128 people diagnosed with HCC in the present investigation, consisting of 721 (64%) who survived, and 407 (36%) who died. The 1-, 3- and 5-year OS rates were 86.3% [95% confidence interval (CI): 86.1–89.9%], 65.3% (95% CI: 62.0–68.9%), and 43.1% (95% CI: 38.4–48.2%), respectively. There were statistically significant variations among the surviving and deceased categories within age, HCC screening status, hepatitis C virus (HCV) status, nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD) status, hypertension status, elevated AFP level, Child-Pugh classification (10), tumor size (maximal diameter of a single tumor), number of tumors, therapy method, portal vein tumor thrombosis (PVTT), and HCC metastasis (P<0.05). There were no notable variables in other factors (P>0.05; Tables 1,2, and Figure 1).
Table 1
| Variables | Overall (n=1,128) | Survival (n=721) | Death (n=407) | t/χ2 | P |
|---|---|---|---|---|---|
| Age (years) | 6.773 | 0.009* | |||
| <55 | 532 (47.16) | 361 (50.07) | 171 (42.01) | ||
| ≥55 | 596 (52.84) | 360 (49.93) | 236 (57.99) | ||
| Sex | 0.285 | 0.59 | |||
| Male | 922 (81.74) | 586 (81.28) | 336 (82.56) | ||
| Female | 206 (18.26) | 135 (18.72) | 71 (17.44) | ||
| Marital status | 0.015 | 0.90 | |||
| Married | 1,091 (96.72) | 697 (96.67) | 394 (96.81) | ||
| Single | 37 (3.28) | 24 (3.33) | 13 (3.19) | ||
| HCC Screening | 40.061 | <0.001* | |||
| No | 961 (85.2) | 578 (80.17) | 383 (94.10) | ||
| Yes | 167 (14.8) | 143 (19.83) | 24 (5.90) | ||
| Smoking | 0.010 | 0.92 | |||
| Yes | 408 (36.17) | 260 (36.06) | 148 (36.36) | ||
| No | 720 (63.83) | 461 (63.94) | 259 (63.64) | ||
| Drinking | 0.753 | 0.39 | |||
| Yes | 337 (29.88) | 209 (28.99) | 128 (31.45) | ||
| No | 791 (70.12) | 512 (71.01) | 279 (68.55) | ||
| Family history of tumors | 0.087 | 0.77 | |||
| Yes | 266 (23.58) | 168 (23.30) | 98 (24.08) | ||
| No | 862 (76.42) | 553 (76.70) | 309 (75.92) | ||
| Therapy method | 212.201 | <0.001* | |||
| LR | 395 (35.02) | 327 (45.35) | 68 (16.71) | ||
| RFA | 84 (7.45) | 71 (9.85) | 13 (3.19) | ||
| LT | 56 (4.96) | 44 (6.10) | 12 (2.95) | ||
| TACE | 332 (29.43) | 156 (21.64) | 176 (43.24) | ||
| TACE + RFA | 86 (7.62) | 58 (8.04) | 28 (6.88) | ||
| TACE + LR | 39 (3.46) | 30 (4.16) | 9 (2.21) | ||
| Expectant treatment | 136 (12.06) | 35 (4.85) | 101 (24.82) | ||
| HCC metastasis | 46.035 | <0.001* | |||
| Yes | 82 (7.27) | 24 (3.33) | 58 (14.25) | ||
| No | 1,046 (92.73) | 697 (96.67) | 349 (85.75) | ||
Data are presented as No. (%). *, P<0.05. HCC, hepatocellular carcinoma; LR, laparoscopic hepatectomy; LT, liver transplantation; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Table 2
| Variables | Overall (n=1,128) | Survival (n=721) | Death (n=407) | t/χ2 | P |
|---|---|---|---|---|---|
| HBV | 3.763 | 0.052 | |||
| Yes | 1,010 (89.54) | 636 (88.21) | 374 (91.89) | ||
| No | 118 (10.46) | 85 (11.79) | 33 (8.11) | ||
| HCV | 5.901 | 0.02* | |||
| Yes | 74 (6.56) | 57 (7.91) | 17 (4.18) | ||
| No | 1,054 (93.44) | 664 (92.09) | 390 (95.82) | ||
| NAFLD | 13.674 | <0.001* | |||
| Yes | 55 (4.88) | 48 (6.66) | 7 (1.72) | ||
| No | 1,073 (95.12) | 673 (93.34) | 400 (98.28) | ||
| ALD | 6.166 | 0.01* | |||
| Yes | 15 (1.33) | 5 (0.69) | 10 (2.46) | ||
| No | 1,113 (98.67) | 716 (99.31) | 397 (97.54) | ||
| Hypertension | 4.401 | 0.04* | |||
| Yes | 226 (20.04) | 158 (21.91) | 68 (16.71) | ||
| No | 902 (79.96) | 563 (78.09) | 339 (83.29) | ||
| Diabetes | 0.220 | 0.64 | |||
| Yes | 159 (14.1) | 99 (13.73) | 60 (14.74) | ||
| No | 969 (85.9) | 622 (86.27) | 347 (85.26) | ||
| Coronary heart disease | 0.162 | 0.69 | |||
| Yes | 33 (2.93) | 20 (2.77) | 13 (3.19) | ||
| No | 1,095 (97.07) | 701 (97.23) | 394 (96.81) | ||
| Cerebral apoplexy† | 0.001 | 0.98 | |||
| Yes | 33 (2.93) | 21 (2.92) | 12 (2.96) | ||
| No | 1,092 (97.07) | 698 (97.08) | 394 (97.04) | ||
| Tumor number | 145.762 | <0.001* | |||
| One | 539 (47.78) | 429 (59.50) | 110 (27.03) | ||
| Two | 68 (6.03) | 56 (7.77) | 12 (2.95) | ||
| Multi | 521 (46.19) | 236 (32.73) | 285 (70.02) | ||
| Tumor size (cm) | 105.258 | <0.001* | |||
| <3 | 296 (26.24) | 238 (33.01) | 58 (14.25) | ||
| 3–5 | 273 (24.2) | 208 (28.85) | 65 (15.97) | ||
| >5 | 559 (49.56) | 275 (38.14) | 284 (69.78) | ||
| AFP (ng/mL) | 50.357 | <0.001* | |||
| <20 | 448 (39.72) | 323 (44.80) | 125 (30.71) | ||
| 20–400 | 282 (25) | 197 (27.32) | 85 (20.88) | ||
| >400 | 339 (30.05) | 176 (24.41) | 163 (40.05) | ||
| Unknown | 59 (5.23) | 25 (3.47) | 34 (8.35) | ||
| Child-Pugh | 66.223 | <0.001* | |||
| A | 870 (77.13) | 611 (84.74) | 259 (63.64) | ||
| B | 228 (20.21) | 99 (13.73) | 129 (31.70) | ||
| C | 30 (2.66) | 11 (1.53) | 19 (4.67) | ||
| PVTT | 38.188 | <0.001* | |||
| Yes | 211 (18.71) | 96 (13.31) | 115 (28.26) | ||
| No | 917 (81.29) | 625 (86.69) | 292 (71.74) |
Data are presented as No. (%). †, three missing data. *, P<0.05. AFP, alpha-fetoprotein; ALD, alcoholic liver disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; PVTT, portal vein tumor thrombosis.
Univariate and multivariate Cox regression analysis
Univariate Cox regression study showed that age, HCC screening, HCV, NAFLD, and ALD status, liver cirrhosis, Child-Pugh classification, tumor size (maximum diameter of a single tumor), the number of tumors, therapy method, PVTT, and HCC metastasis were prognostic risk variables for OS in patients with HCC (P<0.05). Among these factors, the hazard ratio (HR) for grade C liver function was 3.05 (P<0.001). Multivariate Cox regression analysis showed that HCC screening status, tumor size (maximal diameter of a single tumor), Child-Pugh classification, and therapeutic approach were separate risk variables for the HCC prognosis (P<0.05, Table 3).
Table 3
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | 1.01 | 1–1.02 | 0.009* | 1.01 | 1–1.02 | 0.17 | |
| HCC screening (vs. yes) | |||||||
| No | 3.13 | 2.07–4.72 | <0.001* | 1.86 | 1.22–2.85 | 0.004* | |
| Therapy method (vs. LR) | |||||||
| RFA | 0.82 | 0.46–1.49 | 0.52 | 0.83 | 0.46–1.53 | 0.56 | |
| LT | 1.27 | 0.68–2.34 | 0.45 | 0.74 | 0.39–1.42 | 0.37 | |
| TACE | 3.43 | 2.59–4.54 | <0.001* | 1.75 | 1.26–2.42 | 0.001* | |
| TACE + RFA | 1.81 | 1.17–2.82 | 0.008* | 1.31 | 0.81–2.10 | 0.27 | |
| TACE + LR | 1.07 | 0.53–2.14 | 0.85 | 1.12 | 0.56–2.27 | 0.75 | |
| Expectant treatment | 7.17 | 5.27–9.76 | <0.001* | 2.77 | 1.91–4.00 | <0.001* | |
| HCC metastasis (vs. yes) | |||||||
| No | 0.36 | 0.27–0.47 | <0.001* | 0.7 | 0.29–1.65 | 0.41 | |
| HCV (vs. yes) | |||||||
| No | 1.69 | 1.04–2.75 | 0.04* | 1.22 | 0.74–2.02 | 0.43 | |
| NAFLD (vs. yes) | |||||||
| No | 2.66 | 1.26–5.61 | 0.01* | 1.26 | 0.59–2.7 | 0.56 | |
| ALD (vs. yes) | |||||||
| No | 0.47 | 0.25–0.89 | 0.02* | 0.47 | 0.24–0.91 | 0.03* | |
| Liver cirrhosis (vs. yes) | |||||||
| No | 0.53 | 0.3–0.95 | 0.03* | 0.93 | 0.52–1.68 | 0.82 | |
| Tumor number (vs. one) | |||||||
| Two | 1.04 | 0.57–1.88 | 0.91 | 1.28 | 0.57–2.89 | 0.55 | |
| Multi | 3.57 | 2.86–4.45 | <0.001* | 1.43 | 0.83–2.47 | 0.19 | |
| Tumor size (cm) (vs. <3) | |||||||
| 3–5 | 1.26 | 0.89–1.80 | 0.19 | 1.17 | 0.81–1.70 | 0.39 | |
| >5 | 3.77 | 2.84–5.01 | <0.001* | 2.47 | 1.71–3.55 | <0.001* | |
| AFP (ng/mL) (vs. <20) | |||||||
| 20–400 | 1.24 | 0.94–1.63 | 0.13 | 1.07 | 0.81–1.42 | 0.63 | |
| >400 | 2.19 | 1.74–2.77 | <0.001* | 1.25 | 0.97–1.63 | 0.09 | |
| Unknown | 1.92 | 1.31–2.81 | 0.001* | 1.23 | 0.83–1.82 | 0.31 | |
| Child-Pugh (vs. A) | |||||||
| B | 2.38 | 1.92–2.94 | <0.001* | 1.83 | 1.46–2.29 | <0.001* | |
| C | 3.05 | 1.91–4.86 | <0.001* | 2.36 | 1.83–3.78 | <0.001* | |
| PVTT (vs. yes) | |||||||
| No | 0.43 | 0.34–0.53 | <0.001* | 0.83 | 0.49–1.40 | 0.49 | |
*, P<0.05. AFP, alpha-fetoprotein; ALD, alcoholic liver disease; CI, confidence interval; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio; LR, laparoscopic hepatectomy; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; OS, overall survival; PVTT, portal vein tumor thrombosis; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Nomogram for predicting survival time of patients with HCC
The HCC nomogram model included five independent risk factors for HCC screening status, tumor size (maximal diameter of a single tumor), ALD status, Child-Pugh classification, and therapy method (Figure 2). In the HCC nomogram prognostic model, the assignment of conservative treatment was 10 points, and the treatment method accounted for the highest proportion of prognostic factors. Tumor size (maximal diameter of a single tumor) >5 cm was assigned a score of 6.25 points, whereas Child-Pugh class B and lack of screening were both assigned a score of 4.5. In addition, ALD had a score of approximately 3, which was a relatively low prognostic factor. Assuming that the patient with HCC was not screened, had ALD, was treated with radiofrequency ablation (RFA), had Child-Pugh class B, and had a single tumor alongside a maximal diameter of 3–5 cm, their OS prognostic assignment would be 15 points. In the nomogram, the 1-, 3-, and 5-year survival rates were 85%, 60%, and 35%, respectively (Figure 2).

Statistical independence validation was conducted to assess the relationship among five indicators. The variance inflation factor (VIF) for each indicator was evaluated, and the results showed that VIF <2 for all the indicators, indicating no significant multicollinearity among them.
Validation of nomogram for predicting OS in patients with HCC
The area under the ROC curve (AUC) of the nomogram established using the statistically significant demographic and clinicopathological indicators in the Cox multivariate analysis was 0.868. The DCA of the constructed model was in the upper right quadrant, suggesting that the model’s capacity to make predictions was more accurate. To compare the defects of the nomogram in predicting survival prognosis, the consistency evaluation index (C-index) was internally verified to obtain the final value.
Greater accuracy in prognosis prediction was indicated by greater numbers. The outcomes demonstrated that the prognostic nomogram’s C-index to forecast OS was 0.808 [95% CI (0.785–0.830)]. Furthermore, the calibration curves of the 1-, 3-, and 5-year OS time were nearly in line with the rational 45° dashed line, demonstrating the model’s strong capacity for prediction (Figure 3).

Discussion
To build an effective and methodical nomogram for forecasting the prognosis for HCC survival, we gathered clinical data from 1,128 patients with HCC in an actual clinical environment for this study. A multivariate Cox regression analysis showed that five independent risk variables affecting survival prognosis could be used as variables in the nomogram. ROC and calibration curves as well as DCA tests determined that the nomogram showed good accuracy. Multifactorial Cox regression analysis showed that HCC screening, maximal diameter of a single tumor, presence of ALD, Child-Pugh classification, and mode of therapy were separate risk variables influencing OS in HCC patients. The HR for grade C liver function indicated that patients alongside grade C liver function had a higher mortality risk ratio.
Studies have shown that poor adherence to screening in those with a high risk for liver cancer is one of the major risk indicators influencing the long-term survival rate of these patients. Furthermore, 70–80% of patients are diagnosed in the medium to late phases due to the poor likelihood of early detection (11). In healthcare systems with timely access to curative therapies (e.g., surgical resection and liver transplantation), early detection is strongly associated with improved survival by enabling interventions before disease progression (12,13). This is particularly evident in regions where multidisciplinary HCC management pathways are well-coordinated (14).
Multifactorial regression analysis in this study demonstrated that the HR for screening indicated a substantial influence of HCC screening on patient survival. Recently, China’s public health emphasis has been focused on grassroots liver cancer screening. The “14th Five-Year Plan for National Health” further proposes expanding coverage of early cancer diagnosis and treatment through multiple channels and directing localities to perform opportunistic screening for key cancers according to the real circumstances (15,16). This measure is conducive to the early detection of HCC and the survival prognosis of patients.
Primary screening for liver cancer in China is based mainly on serum AFP detection and liver ultrasound examination. China developed the whole blood one-step procedure for the first home AFP test strip. This method achieved good feedback in liver cancer screening because of its simplicity, convenience, high positive detection rate, and high diagnostic specificity (17). However, considering the insufficient diagnostic ability of a single indicator of liver cancer, Tzartzeva et al. (18) have attempted to combine these two biomarkers for improved liver cancer detection.
Wang et al. (19) have shown that the diagnostic value of combining AFP-L3% or des-gamma-carboxyprothrombin (DCP) with AFP is significantly higher than that of a single test for liver cancer diagnosis. However, according to Wu et al.’s study (20), adding AFP-L3% or DCP to AFP does not much increase the HCC diagnostic value. To date, only Japan has approved AFP in combination with AFP-L3% and DCP as a tumor marker for routine liver cancer screening (21,22). To sum up, more research with a “higher level of evidence” is required in the future to verify the degree to which biomarker combination testing enhances the usefulness of HCC screening. This would also contribute to identifying the optimal combination of markers to assist physicians and patients in making the best decisions.
Previous national and international studies have shown controversial results on the potential impact of tumor size (maximal diameter of a single tumor) on survival prognosis (23,24). A multicenter investigation revealed that in those suffering from HCC, a single tumor’s maximal diameter of more than 5 cm was a separate risk indicator for a bad prognosis (25). Another research determined by Surveillance, Epidemiology, and End Results (SEER) data analyzed the dose-response relationship between the maximum diameter of individual tumors and the prognosis of HCC survival (26). Another study used a restricted cubic bar-like model, and the results showed a nonlinear dose-response relationship (27). This study used multifactorial Cox regression evaluation to identify a single tumor with a maximal diameter >5 cm as a poor prognostic indicator for survival in HCC.
Notably, our results challenge the biological homogeneity assumed by the Barcelona Clinic Liver Cancer (BCLC) staging system for tumors >2 cm. While BCLC classifies all tumors exceeding 2 cm into advanced stages (BCLC-B/C) (28), our data suggest significant heterogeneity among larger tumors. Specifically, tumors >5 cm (HR =2.47, P<0.001) exhibited distinct survival outcomes compared to those ≤5 cm (HR =1.17, P=0.39), implying that size alone beyond 2 cm may inadequately reflect tumor aggressiveness. This discrepancy aligns with recent study demonstrating that size >5 cm independently correlates with microvascular invasion and genomic instability (29), factors not directly captured by BCLC’s current framework. Notably, the 2022 BCLC update acknowledges this limitation and calls for integrating auxiliary biomarkers (e.g., AFP, radiomics) to refine staging (30), a strategy our nomogram partially addresses through multivariable risk modeling.
ALD is a liver disease caused by excessive and sustained alcohol consumption. While moderate drinkers also have a greater risk of HCC compared to non-drinkers, heavy drinkers have a 2.07 times greater risk of HCC than that of non-drinkers (31). Several studies have reported that the prognosis for survival in patients without ALD is better than that in patients with ALD (32,33). In this study, Cox multifactorial regression analysis determined that ALD has a significant influence on HCC survival likelihood. Furthermore, the HR of the group without ALD indicated that its absence was a protective factor, improving the survival prognosis and quality of life for patients with HCC.
In addition to other liver function evaluation techniques, the Child-Pugh clinical grading scale is frequently used to objectively evaluate hepatic reserve function in patients with cirrhosis. It is used to guide subsequent treatment and prognosis in HCC and as one of the baseline indicators in the assessment and diagnosis. Ding et al. (26) have shown a strong association between Child-Pugh classification and survival prognosis in patients with HCC. In this study, a Child-Pugh B classification had an HR that was indicative of a risk variable for survival in patients with HCC and it had a worse prognosis for survival than that of a grade A classification.
Recently, HCC treatment has mainly been based on different clinical stages and pathological types, using different treatment protocols. Hepatectomy, liver transplantation, radiation therapy, ablation therapy, transarterial chemoembolization (TACE), and systemic anticancer therapy are among the common therapeutic modalities. Therapeutic results are maximized when individuals who have liver cancer at various phases receive appropriate therapy (30).
National and international studies have shown that whether palliative or radical surgery is selected could improve the prognosis to different degrees (34). The results of similar earlier research are consistent with the findings of the current investigation, which found that TACE alone was not as clinically beneficial as TACE coupled with RFA in the therapy of HCC (35). With a high score on the column line graph, the study’s findings indicated that the lack of surgical therapy was a distinct risk indicator for the prognosis of survival.
Conclusions
Compared with most previous HCC retrospective analyses and prognostic model construction studies, the method used in this study has several advantages. Based on a relatively large number of real clinical information, combined with clinical and pathological characteristics and a wide range of demographic characteristics, we analyzed the factors affecting the OS of HCC patients, and established a relatively accurate survival prediction model for HCC patients. In contrast to BCLC, it can provide specific scientific direction for the prognosis of patients, reflect the regional characteristics of HCC development in China, and have certain reference function.
However, there are some limitations in this study that are worth mentioning. Initially, this was a small-sample, single-center research with a single source of data, which lacks generality and representativeness and requires a large amount of multi-center data for external validation. Second, we explored the relationship between multiple independent risk factors and the survival and prognosis of patients with HCC. The prognostic value of each independent risk factor needs to be explored further, which may be a direction for future research.
In summary, there was no screening for HCC, and tumor size (single tumor diameter) >5 cm, ALD, Child-Pugh classification C, and conservative treatment were independent risk variables for OS in patients with HCC. Clinicians may use a nomogram based on these independent risk variables as a guide for determining the survival prognosis of patients with HCC, since it correctly, intuitively, and individually predicted the OS of these individuals.
Acknowledgments
We grateful to our colleagues for their assistance in checking the data.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-806/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-806/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-806/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-806/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Zhengzhou University (No. ZZUIRB2022-151) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Liu Y, Yang L, Yu M, et al. Construction of a ceRNA network to reveal a vascular invasion associated prognostic model in hepatocellular carcinoma. Open Med (Wars) 2023;18:20230795. [Crossref] [PubMed]
- Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156:477-491.e1. [Crossref] [PubMed]
- Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77:1598-606. [Crossref] [PubMed]
- Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. [Crossref] [PubMed]
- Han T, Zhou Y, Li D. Relationship between hepatocellular carcinoma and depression via online database analysis. Bioengineered 2021;12:1689-97. [Crossref] [PubMed]
- Zhang P, Feng J, Wu X, et al. Bioinformatics Analysis of Candidate Genes and Pathways Related to Hepatocellular Carcinoma in China: A Study Based on Public Databases. Pathol Oncol Res 2021;27:588532. [Crossref] [PubMed]
- Fujiwara N, Friedman SL, Goossens N, et al. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol 2018;68:526-49. [Crossref] [PubMed]
- Levaillant M, Marcilly R, Levaillant L, et al. Assessing the hospital volume-outcome relationship in surgery: a scoping review. BMC Med Res Methodol 2021;21:204. [Crossref] [PubMed]
- Tsoris A, Marlar CA. Use Of The Child Pugh Score In Liver Disease. 2025.
- Guo Z, Wang J, Li L, et al. Value of miR-1271 and glypican-3 in evaluating the prognosis of patients with hepatocellular carcinoma after transcatheter arterial chemoembolization. World J Clin Cases 2020;8:3493-502. [Crossref] [PubMed]
- Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;77:128-39. [Crossref] [PubMed]
- Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706-1718.e1. [Crossref] [PubMed]
- 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583-705. [Crossref] [PubMed]
- Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer 2023;12:405-44. [Crossref] [PubMed]
- Xu Y, Xia C, Li H, et al. Survey of hepatitis B virus infection for liver cancer screening in China: A population-based, cross-sectional study. Chin Med J (Engl) 2024;137:1414-20. [Crossref] [PubMed]
- Huang D, Zhang J, Xu J, et al. Utility of Alpha-Fetoprotein and Ultrasound in the Diagnosis and Prognosis of Patients with Hepatocellular Liver Cancer. J Multidiscip Healthc 2024;17:1819-26. [Crossref] [PubMed]
- Tzartzeva K, Singal AG. Testing for AFP in combination with ultrasound improves early liver cancer detection. Expert Rev Gastroenterol Hepatol 2018;12:947-9. [Crossref] [PubMed]
- Wang X, Zhang Y, Yang N, et al. Evaluation of the Combined Application of AFP, AFP-L3%, and DCP for Hepatocellular Carcinoma Diagnosis: A Meta-analysis. Biomed Res Int 2020;2020:5087643. [Crossref] [PubMed]
- Wu M, Liu Z, Li X, et al. Dynamic Changes in Serum Markers and Their Utility in the Early Diagnosis of All Stages of Hepatitis B-Associated Hepatocellular Carcinoma. Onco Targets Ther 2020;13:827-40. [Crossref] [PubMed]
- Fang YS, Wu Q, Zhao HC, et al. Do combined assays of serum AFP, AFP-L3, DCP, GP73, and DKK-1 efficiently improve the clinical values of biomarkers in decision-making for hepatocellular carcinoma? A meta-analysis. Expert Rev Gastroenterol Hepatol 2021;15:1065-76. [Crossref] [PubMed]
- Mehta N, Kotwani P, Norman J, et al. AFP-L3 and DCP are superior to AFP in predicting waitlist dropout in HCC patients: Results of a prospective study. Liver Transpl 2023;29:1041-9. [Crossref] [PubMed]
- Li JD, Xu XF, Han J, et al. Preoperative prealbumin level as an independent predictor of long-term prognosis after liver resection for hepatocellular carcinoma: a multi-institutional study. HPB (Oxford) 2019;21:157-66. [Crossref] [PubMed]
- Usta S, Kayaalp C. Tumor Diameter for Hepatocellular Carcinoma: Why Should Size Matter? J Gastrointest Cancer 2020;51:1114-7. [Crossref] [PubMed]
- Zhang W, Jin K, Wang F, et al. Differences in the prognostic value of tumor size on hepatocellular cancer-specific survival stratified by gender in a SEER population-based study. United European Gastroenterol J 2019;7:933-41. [Crossref] [PubMed]
- Ding J, Wen Z. Survival improvement and prognosis for hepatocellular carcinoma: analysis of the SEER database. BMC Cancer 2021;21:1157. [Crossref] [PubMed]
- Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol 2019;70:284-93. [Crossref] [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [Crossref] [PubMed]
- Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res 2021;149:1-61. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Malnick SDH, Alin P, Somin M, et al. Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different. Int J Mol Sci 2022;23:16226. [Crossref] [PubMed]
- Liu SY, Tsai IT, Hsu YC. Alcohol-Related Liver Disease: Basic Mechanisms and Clinical Perspectives. Int J Mol Sci 2021;22:5170. [Crossref] [PubMed]
- Leal CRG, Magalhães C, Barbosa D, et al. Survival and tolerance to sorafenib in Child-Pugh B patients with hepatocellular carcinoma: a prospective study. Invest New Drugs 2018;36:911-8. [Crossref] [PubMed]
- Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64:106-16. [Crossref] [PubMed]
- Zhou XH, Li JR, Zheng TH, et al. Portal vein tumor thrombosis in hepatocellular carcinoma: molecular mechanism and therapy. Clin Exp Metastasis 2023;40:5-32. [Crossref] [PubMed]


