Malignant transformation of biliary adenofibroma: a rare biliary cystic tumor
Introduction
Biliary adenofibromas (BAFs) are rare, benign biliary cystic tumors with uncommon potential for malignant transformation (1,2). To our knowledge, only 11 cases of BAF have been reported in the literature (1-12). We performed a database search to find cases of BAF diagnosed at Mayo Clinic from years 1994 to 2016. We describe two cases of malignant BAF.
Case presentation
Case 1
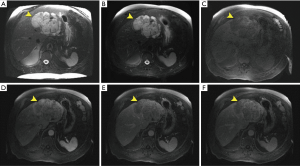
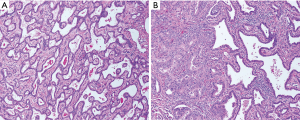
A 71-year-old male was referred to a hepatologist for evaluation of a large mass in the left lateral hepatic lobe, discovered incidentally during screening ultrasound (US) for an abdominal aortic aneurysm (AAA). At the time of evaluation, the patient denied weight loss, fever, chills or jaundice. The patient was a chronic smoker and consumed two drinks per day. The remaining past medical, surgical, family and social history were non-contributory. The patient was obese with a body mass index (BMI) of 38.8 kg/m2 with an otherwise unremarkable physical examination; there were no stigmata of chronic liver disease. Tumor markers including AFP and CA 19-9 were not elevated. Liver function tests were within normal limits and serology was negative for hepatitis B virus (HBV) and hepatitis C virus (HCV) infection. Magnetic resonance imaging (MRI) was performed, which showed a 14.5 cm × 10 cm × 6.3 cm complex mass with both solid and cystic components confined to the left hepatic lobe (Figure 1). There were two enlarged periportal lymph nodes measuring 1 cm concerning for metastasis (not shown). The remainder of the liver had a normal morphologic appearance. Staging studies including a PET/CT scan (positron emission tomography/computed tomography) and esophagogastroduodenoscopy (EGD) with endoscopic ultrasound (EUS) were negative for extrahepatic disease. Review of the outside liver fine needle aspiration (FNA) suggested an epithelial neoplasm with glandular and papillary architecture and mild cytologic atypia suggestive of a low grade adenocarcinoma, possibly of biliary origin, and morphologic features compatible with an adenofibroma. The patient underwent a left hepatectomy, which contained an 11 cm × 10.5 cm × 6 cm soft pink mass encompassing a 9 cm × 3.5 cm × 3 cm firm, white mass. The larger component showed irregularly shaped spaces lined by a single layer of bland, cuboidal epithelium (Figure 2A). The firm white area harbored a moderately differentiated adenocarcinoma (Figure 2B). A single regional lymph node was negative for tumor. The patient did not develop any recurrence of his malignancy. He passed away nine years later from a new primary lung malignancy.
Case 2
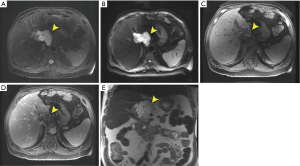
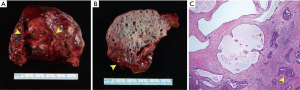
A 71-year-old male was referred to a hepatologist for evaluation of a 5.7-cm caudate lobe mass incidentally discovered on a CT scan. At the time of evaluation, the patient denied weight loss, fever, chills or jaundice. There was no relevant past medical, surgical, family or social history. The patient was overweight with a BMI of 29 kg/m2 with an unremarkable physical examination. Tumor markers including AFP, CA 19-9 and CEA were not elevated. Liver function tests were within normal limits and he had no serologic evidence of HBV or HCV infection. MRI was performed, which showed a 6.6 cm × 6.3 cm multilobulated, multiseptated cystic mass confined to the caudate lobe (Figure 3). There was an enlarged periportal lymph node measuring 3.3 cm concerning for metastasis (not shown). The remainder of the liver had a normal morphologic appearance. The leading diagnosis by imaging was a biliary cystadenocarcinoma and a pre-operative core needle biopsy suggested this diagnosis. The patient underwent surgical resection of the caudate lobe. The multicystic tumor measured 6.3 cm and had subtle transitions to firm gray-red areas laterally (Figure 4A,B). The cystic spaces were lined by low cuboidal epithelium, while the solid areas contained an invasive well-differentiated adenocarcinoma (Figure 4C). Eleven regional lymph nodes were negative for tumor. The tumor cells were positive for CK7 and negative for CDX-2 and CK20. The patient has had no evidence of local recurrence by CT at first four-week follow-up. Next generation sequencing of the tumor identified a loss of function (nonsense) mutation (Q50*) in the tumor suppressor protein p16INK4a encoded by the cyclin-dependent kinase inhibitor 2A (CDKN2A) gene.
Discussion
BAF are rare, benign biliary cystic tumors characterized histopathologically by: (I) non-mucin producing, biliary epithelium; (II) tubulocystic architecture; (III) fibrous stroma; and (IV) rare potential for malignant transformation (1). Given their potential for malignant transformation, BAFs are hypothesized to be a precursor to peripheral intrahepatic cholangiocarcinoma (1). Of the eleven prior cases of BAF reported in the literature, six were associated with evidence of malignant transformation (Table 1) (1-12).
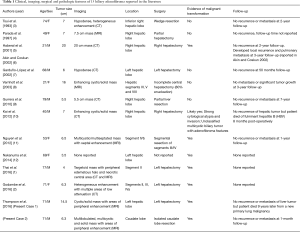
Full table
The imaging findings of a large, liver mass with both cystic and enhancing solid components in a patient without underlying liver disease are consistent with a complex cystic liver lesion and a biliary cystic tumor is to be considered at the top of the differential. The differential diagnosis for biliary cystic tumors includes both benign (e.g., biliary cystadenoma and BAF) and malignant tumors (e.g., biliary cystadenocarcinoma and the malignant transformation of a benign BAF). In our series, the complex cystic appearance and enlarged periportal lymph nodes on imaging were suggestive of a malignant rather than a benign neoplasm, such as a biliary cystadenocarcinoma. However, imaging alone cannot reliably differentiate biliary cystadenoma from cystadenocarcinoma (13). Moreover, the reports that include imaging findings of malignant BAF are similar to other biliary cystic tumors (1-11,13). As such, a tissue diagnosis is needed. Of note, a pre-operative FNA biopsy in the first patient showed an adenocarcinoma, possibly arising in a background of BAF, whereas the biopsy in the second patient was suggestive of a biliary cystadenocarcinoma. However, in both patients, final surgical pathology showed adenocarcinoma arising in a background of BAF, further highlighting the difficulty in making this diagnosis with limited tissue.
Furthermore, it appears to be uncommon for these tumors to metastasize or recur after surgical resection (1,2,10-12). Only one prior report of a malignant BAF developed local recurrence and pulmonary metastasis three years after surgical resection (5,6). Our cases suggest that recurrence or metastasis of benign or malignant BAF is not common, even with large tumors, and that complete surgical resection can be curative (Table 1). Nonetheless, longer follow-up is needed. Lastly, the CDKN2A mutation identified in the second patient has previously been implicated in the pathogenesis of biliary dysplasia and cholangiocarcinoma and this is the first report of a CDKN2A mutation in a malignant BAF (14). In summary, these two cases highlight that malignant BAF, although rare, should be considered in the differential diagnosis of a complex cystic liver mass consistent with a biliary cystic tumor and may share a similar molecular pathogenesis to other biliary tract malignancies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: This HIPAA-compliant study was approved by the Institutional Review Board and a waiver of informed consent was granted.
References
- Thai E, Dalla Valle R, Evaristi F, et al. A case of biliary adenofibroma with malignant transformation. Pathol Res Pract 2016;212:468-70. [Crossref] [PubMed]
- Godambe A, Brunt EM, Fulling KH, et al. Biliary Adenofibroma with Invasive Carcinoma: Case Report and Review of the Literature. Case Rep Pathol 2016;2016:8068513.
- Tsui WM, Loo KT, Chow LT, et al. Biliary adenofibroma. A heretofore unrecognized benign biliary tumor of the liver. Am J Surg Pathol 1993;17:186-92. [Crossref] [PubMed]
- Parada LA, Bardi G, Hallén M, et al. Monosomy 22 in a case of biliary adenofibroma. Cancer Genet Cytogenet 1997;93:183-4. [Crossref] [PubMed]
- Haberal AN, Bilezikçi B, Demirhan B, et al. Malignant transformation of biliary adenofibroma: A case report. Turk J Gastroenterol 2001;12:149-53.
- Akin O, Coskun M. Biliary adenofibroma with malignant transformation and pulmonary metastases: CT findings. AJR Am J Roentgenol 2002;179:280-1. [Crossref] [PubMed]
- Garduño-López AL, Mondragón-Sánchez R, Bernal-Maldonado R, et al. A case of biliary adenofibroma of the liver causing elevated serum CA 19-9 levels. Rev Oncol 2002;4:271-3.
- Varnholt H, Vauthey JN, Dal Cin P, et al. Biliary adenofibroma: a rare neoplasm of bile duct origin with an indolent behavior. Am J Surg Pathol 2003;27:693-8. [Crossref] [PubMed]
- Gurrera A, Alaggio R, Leone G, et al. Biliary adenofibroma of the liver: report of a case and review of the literature. Patholog Res Int 2010;2010:504584.
- Kai K, Yakabe T, Kohya N, et al. A case of unclassified multicystic biliary tumor with biliary adenofibroma features. Pathol Int 2012;62:506-10. [Crossref] [PubMed]
- Nguyen NT, Harring TR, Holley L, et al. Biliary adenofibroma with carcinoma in situ: a rare case report. Case Reports Hepatol 2012;2012:793963.
- Nakanuma Y, Tsutsui A, Ren XS, et al. What are the precursor and early lesions of peripheral intrahepatic cholangiocarcinoma? Int J Hepatol 2014;2014:805973.
- Soares KC, Arnaoutakis DJ, Kamel I, et al. Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma. J Am Coll Surg 2014;218:119-28. [Crossref] [PubMed]
- DeHaan RD, Kipp BR, Smyrk TC, et al. An assessment of chromosomal alterations detected by fluorescence in situ hybridization and p16 expression in sporadic and primary sclerosing cholangitis-associated cholangiocarcinomas. Hum Pathol 2007;38:491-9. [Crossref] [PubMed]


