Successful hepatectomy for metastatic squamous cell carcinoma of the anal canal—a case report
Introduction
Despite being relatively rare tumors, the incidence of anal cancer in both men and women has been increasing over the past 30 years. Changing trends in sexual behavior, smoking, infection with human papillomavirus (HPV), immune suppression in transplant recipients, use of immunosuppressants such as long-term corticosteroids, and infection with human immunodeficiency virus (HIV) are thought to be responsible for the rise (1). The majority of cases present with early-stage localized disease, which is associated with a good prognosis (2). In this setting combined modality treatment with chemoradiation (CRT) remains the standard of care allowing sphincter sparing whilst reserving surgery for those with persistent or recurrent disease following treatment (3-6). However, five-year overall survival (OS) for those with more locally advanced disease ranges from 40% to 80% and approximately 10% to 20% of patients suffer distant metastases relapse following definitive CRT (7,8). Systemic spread is uncommon with distant extra-pelvic metastases reported in 5% to 8% at initial diagnosis. Unfortunately, the prognosis for patients with metastatic disease remains poor with an estimated five-year OS of only 19% (9).
The most common sites of metastatic spread in anal cancers are to the para-aortic nodes, liver, lungs and skin, which usually appear late and in the context of local persistent or recurrent disease (10). When compared to isolated liver colorectal or neuroendocrine cancer liver metastases, there is far less experience with resection or nonsurgical local ablative procedures for patients with metastatic anal carcinoma to the liver.
Here, we report on a patient with metastatic anal cancer to the liver who was successfully treated with liver resection and remains free of relapse.
Case presentation
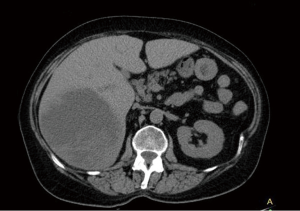
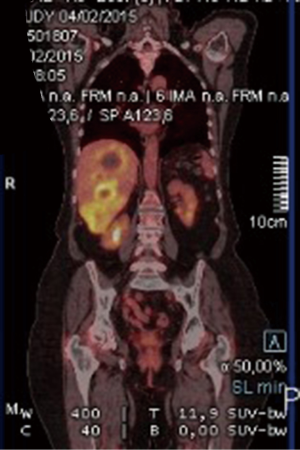
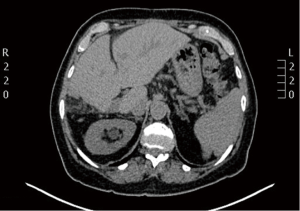
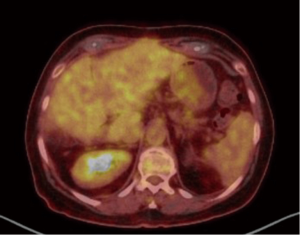
A 67-year-old Caucasian female presented in June 2013 with a 3-month history of rectal bleeding associated with tenesmus. Digital exam revealed a 5 cm lesion at the anal canal at the level of the dentate line. CEA and CA19.9 were within normal values and HIV testing was negative. Anoscopy-guided biopsy confirmed a poorly-differentiated squamous-cell carcinoma of the anus. Local staging was assessed by magnetic resonance imaging (MRI) of the pelvis, which showed a 5.1-cm anal canal lesion as well as enlarged bilateral inguinal lymph nodes (2.0 cm), consistent with a clinical stage IIIB. At that time, the patient was treated with standard chemoradiation (CRT) consisting of mitomycin C (12 mg/m2, given on day 1) and Capecitabine (825 mg/m2 twice daily during the days of radiotherapy). The total radiation therapy dose was 54 Gy in 27 fractions. During CRT, she developed grade 3 cutaneous radiation dermatitis and grade 3 diarrhea. Clinical examination four weeks after finishing CRT revealed complete regression of the anal tumor with no palpable inguinal lymphadenopathy. Nevertheless, 4 months after the completion of CRT, she developed at least three metastatic liver lesions in segments V and VI, measuring approximately 3.6 cm each. She was treated with 6 cycles of weekly Paclitaxel (80 mg/m2) and Cetuximab (400 mg/m2 in the first cycle followed by 250 mg/m2). A repeat CT scan as well as a PET-CT scan revealed considerable increase in the size of the larger liver mass, which measured 13.1 cm × 10.7 cm × 10.6 cm, without new metastases (Figures 1 and 2). At that point, the patient was complaining of intense fatigue, anorexia, as well as upper abdominal pain. She was confined to bed with an extremely poor quality of life. Her lactate dehydrogenase (LDH) level was 1,045 U/L (normal values: 180–460 U/L). After extensive discussion with the patient and her family, it was decided to proceed with right hepatectomy. The postoperative period was uncomplicated and the patient was discharged home six days later. Histological findings of the resected specimen demonstrated metastatic squamous cell carcinoma with extensive necrosis, consistent with the known primary tumor of the anal canal, with free margins. Thirteen months after surgery, the patient remains remarkably well and free of recurrence (Figures 3 and 4).
Discussion
In patients with primary tumors of the gastrointestinal tract (especially colorectal adenocarcinoma and small intestine neuroendocrine tumors), the most likely mode of spread to the liver is through portal venous drainage or via direct intra-abdominal lymphatic channels. For other primary malignancies, metastases commonly reach the liver via systemic circulation, implying that extra-hepatic sites may have the same probability of being involved. Based on this rationale, hepatic resection of non-colorectal and non-neuroendocrine liver metastases has been approached with more caution.
It is known for decades that selected patients with resectable metastatic colorectal cancer isolated to the liver may safely undergo hepatectomy reaching 5-year OS beyond that expected with chemotherapy alone. Surgical resection is actually curative (≥10-year DFS) in at least 17% of patients with metastatic colorectal cancer to the liver (11). As a result, hepatic resection has become the standard of care for patients with resectable metastases from colorectal origin. In addition, the role of surgery for metastases from neuroendocrine neoplasms on long-term outcome is also well-established (12). On the other hand, the liver is a common site of metastatic disease for several other malignancies while there is a paucity of data in the medical literature regarding the benefits for hepatectomy in this group.
Systemic chemotherapy remains the mainstay of treatment for patients with metastatic anal carcinoma while the role of biologics and/or surgical resection of metastatic disease are anecdotal. To the best of our knowledge, there is only one prior case report on a patient with metastatic squamous cell carcinoma of the anal canal who derived benefit from hepatectomy. According to the authors, a 54-year-old woman presented with squamous cell anal carcinoma and concomitant liver metastases. She was treated with a combination of CRT for the primary tumor and then underwent surgery for liver metastases. Two and 5 years after presentation, the patient underwent repeated partial hepatectomies for recurrent liver disease. Five months after completing therapy and 71 months after the initial diagnosis, she remained in good health with no evidence of disease (13).
In parallel, the role of regional therapies in patients with limited metastatic disease was assessed in a study evaluating liver resection, ablation, or both in a group of 52 patients with metastatic squamous cell carcinoma. In the subgroup of 27 patients with anal origin, DFS and OS were 9.6 and 22.3 months, respectively. Although small, this study suggests that long-term survival can be achieved following surgical resection. Regional therapy may, therefore, be an option for individuals with metastatic squamous cell carcinoma of the anus if there is meticulous patient selection.
The particularity of this case lays on the fact that the patient was extremely symptomatic from the liver mass, had progressed through palliative chemotherapy and was confined to bed with a poor quality of life. Surgical resection had led not only to disappearance of all her symptoms but also to complete remission. It illustrates the role of hepatectomy for symptomatic relief and raises the contribution of metastasectomy to provide a chance of cure.
Although systemic chemotherapy plays the key role in the therapy of patients with metastatic anal carcinoma, surgery may act as an adjuvant treatment in selected cases. Patients with isolated metastatic disease should have their cases further discussed by an appropriate multidisciplinary team for consideration of metastasectomy. Randomized trials are needed to help answering the question whether surgery has a definitive role in the management of metastatic anal carcinoma similarly to colorectal cancer.
In conclusion, hepatectomy for liver metastases from anal cancer appears beneficial and should be further investigated in randomized trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Greenall MJ, Quan SH, Stearns MW, et al. Epidermoid cancer of the anal margin. Pathologic features, treatment, and clinical results. Am J Surg 1985;149:95-101. [Crossref] [PubMed]
- Allal A, Kurtz JM, Pipard G, et al. Chemoradiotherapy versus radiotherapy alone for anal cancer: a retrospective comparison. Int J Radiat Oncol Biol Phys 1993;27:59-66. [Crossref] [PubMed]
- Tanum G, Tveit K, Karlsen KO, et al. Chemotherapy and radiation therapy for anal carcinoma. Survival and late morbidity. Cancer 1991;67:2462-6. [Crossref] [PubMed]
- Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996;348:1049-54. [Crossref] [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [PubMed]
- Palefsky JM. Anal human papillomavirus infection and anal cancer in HIV-positive individuals: an emerging problem. AIDS 1994;8:283-95. [Crossref] [PubMed]
- NCCN: Anal Carcinoma National Comprehensive Cancer Network. Available online: http://www.nccn.org/professionals/physician_gls/pdf/anal.pdf
- Ries LA, Young JL, Keel GE, et al. editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
- Bilimoria KY, Bentrem DJ, Rock CE, et al. Outcomes and prognostic factors for squamous-cell carcinoma of the anal canal: analysis of patients from the National Cancer Data Base. Dis Colon Rectum 2009;52:624-31. [PubMed]
- Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol 2014;111:330-9. [Crossref] [PubMed]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575-80. [Crossref] [PubMed]
- Jagannath P, Chhabra D, Shrikhande S, et al. Surgical treatment of liver metastases in neuroendocrine neoplasms. Int J Hepatol 2012;2012:782672.
- Tokar M, Bobilev D, Zalmanov S, et al. Combined multimodal approach to the treatment of metastatic anal carcinoma: report of a case and review of the literature. Onkologie 2006;29:30-2. [PubMed]