Investigating the prognostic value of KOC (K homology domain containing protein overexpressed in cancer) overexpression after curative intent resection of pancreatic ductal adenocarcinoma
Introduction
As of 2016, pancreatic ductal adenocarcinoma (PDAC) is estimated to become the 3rd leading cause of cancer mortality in the United States (1). Effective screening and early detection modalities are currently unavailable, thereby 80% of patients present with distant metastasis. For patients presenting with advanced metastatic disease clinical trial enrollment or palliative chemotherapy remains the standard of care for those with acceptable performance status. To date surgical resection remains the only definitive cure for PDAC. However, alarmingly, even patients with localized disease eligible for surgical resection have a median survival limited to 22.8 months due to high recurrence rates (2). Therefore, 82% of patients diagnosed with PDAC ultimately succumb to their disease (3). Traditionally, negative surgical margins, tumor size, stage, and node negative disease serve as universal prognostic indicators. However, these factors are limited in their ability to predict patient specific prognosis. Therefore, there is an ongoing need to identify individual prognostic and predicative biomarkers in PDAC.
The K homology domain containing protein overexpressed in cancer (KOC) gene, also known as IGF2BP3 is located on chromosome 7p11.5 (4). It encodes a novel oncofetal RNA-binding protein (580 AA) with four internal peptide repeats corresponding to KH domains involved in RNA stabilization and cell growth during embryogenesis (4). It has been known for quite some time that the KOC transcript is highly expressed in pancreatic carcinomas (4,5). Of note, KOC mRNA is inappropriately overexpressed in pancreatic cancer and increased expression correlates with worsening tumor grade (6). It is involved in the regulation of mRNA stability and subcellular localization for fundamental biological processes such as development, cell growth and differentiation. RNA-binding proteins have a wide range of functions, including posttranscriptional regulation of gene expression (7). Interestingly KOC expression appears to be restricted to proliferating tissues, suggesting that KOC may be involved in stabilizing transcripts associated with tumor cell proliferation (8). Overexpression of KOC in various cancer tissues may play a specific role in posttranscriptional regulation of gene expression by interfering with RNA stability.
KOC overexpression has been previously reported to represent a marker of more aggressive disease in urothelial carcinomas of the bladder, renal clear cell carcinoma and ovarian clear cell carcinomas (9-12). In this study, we attempt to identify whether KOC expression in patients with PDAC who undergo curative intent surgery is associated with clinically relevant outcomes, specifically, progression free survival (PFS).
Methods
The expression of KOC was evaluated by immunohistochemistry on a tissue microarray of 62 pancreatic adenocarcinoma (PDAC) specimens from patients who underwent curative intent surgical resection at Geisinger Medical Center from 1998–2011 (Table 1). A retrospective analysis of the available clinical data in regards to patient outcomes was performed. This study was approved by the Geisinger Medical Center Institutional Review Board in Danville, Pennsylvania.
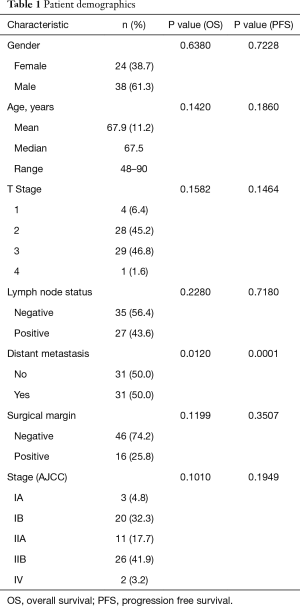
Full table
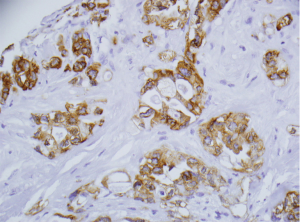
Pathological staging and histological tumor differentiation were determined from the original hematoxylin and eosin stained slides. Tissue microarrays were prepared by our Pathology department from the formalin-fixed, paraffin-embedded specimens of patients. KOC was represented as low and high expression by categorizing the expression score at <4+ or ≥4+, respectively, based on intensity and extent of cells stained (Figure 1). The distribution (number of tumor cells stained for KOC) was recorded as negative (<5% of tumor cells stained), 1+ (5–25%), 2+ (26–50%), 3+ (51–75%), or 4+ (>75%). Two surgical pathologists (Fan Lin, Shaobo Zhu) independently evaluated the immunostained slides.
According to the AJCC (6th edition) classification, the study cohort was distributed as follows: IA (n=3), IB (n=20), IIA (n=11), IIB (n =26), and IV (n=2). Full patient demographics are provided in Table 1. In regards to statistical analyses, comparisons between groups on PFS are estimated using the Kaplan-Meier method and the log-rank test. Statistical significance was declared if the P value was ≤0.05.
Results
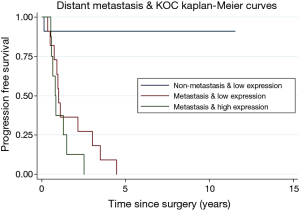
Initial evaluation consisted of only 35 patients to screen for relevance of KOC as a prognostic marker in PDAC. Comparisons between groups on PFS are estimated using the Kaplan-Meier method and the log-rank test. KOC was overexpressed in 23.6% of tumors. It was found that there were zero patients with a high KOC expression and no distant metastasis (Figure 2). Patients with metastatic disease and a high KOC expression were more than 3 times more likely to progress compared to those with a low KOC expression, potentially identifying a biologically distinct subgroup of PDAC (HR =3.54; 95% CI: 1.34, 9.36; P=0.011) (Figure 2).
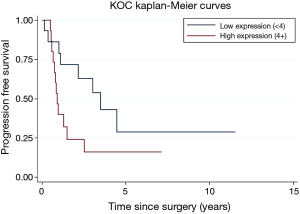
A subsequent updated analysis included a total of 62 total patients. Overall survival (OS) and PFS are estimated using the Kaplan-Meier method and the log-rank test. Forty deaths occurred in the sample. Twenty-nine patients progressed in their disease. Distant metastasis and differentiation (well/moderate vs. poor) were related to OS (P=0.0120, P=0.0086). High/low KOC expression were borderline significantly related to PFS (P=0.0556). Incorporating previously reported data on KOC, patients with a high KOC expression were more than two times more likely to progress compared those with a low KOC expression (HR =2.04; 95% CI: 0.97, 4.29) (Figure 3).
Discussion
Identifying biomarkers of prognostic and/or predictive value is of critical importance in the evolving field of precision gastrointestinal oncology. For instance, in the arena of metastatic colorectal cancer expanded RAS mutational status is now a key predictive marker for response to anti-EGFR therapy and dictates its utilization, while BRAF mutation status has proven to be prognostic. Most recently, negative immunohistochemical expression of the transcription factor CDX2 was retrospectively found to identify a cohort of stage II colorectal cancer patients that have worse clinical outcomes and potentially benefit from adjuvant chemotherapy (13). In early phase clinical trials, a cohort of treatment refractory non-colorectal GI cancer patients, including pancreatic ductal adenocarcinoma, with high microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR) have shown meaningful and durable response to immunotherapy with anti-PD-1 checkpoint blockade (14). Although all of this data is provocative, to date there are no molecular or immunohistochemical biomarkers of clinical significance currently being used to individualize treatment approaches in the management of PDAC, thereby representing a critical area for research development.
In our study, we retrospectively investigated the expression patterns of KOC as a meaningful prognostic biomarker in the tumor tissue of sixty-two PDAC patients who underwent curative intent surgery. KOC expression did in fact correlate with specific clinical outcomes, particularly PFS. Patients with a high KOC expression were more than two times more likely to progress compared to those with a low KOC expression (Figure 3). Our results reveal that KOC appears to be a useful prognostic biomarker for predicting a subset of patients with PDAC who have a high risk for early progression and distant metastasis. Identifying patients with high KOC expression upon initial diagnosis might serve as a way to decipher the underlying biology of a patient’s pancreatic tumor up front, thereby tailoring therapeutic approaches for the individual patient.
The use of KOC expression as a biomarker may prove to be particularly relevant today as current approaches regarding the management of borderline resectable and resectable pancreatic cancer continue to evolve with neoadjuvant therapy. Although neoadjuvant chemotherapy is not yet accepted as a standard of care for borderline resectable or resectable PDAC, the success of systemic combination chemotherapy particularly FOLFIRINOX in the metastatic setting has resulted in numerous clinical trials aimed to validate a neoadjuvant approach (NCT01821612, NCT01660711, NCT02172976, NCT01521702) (15,16). Theoretically, neoadjuvant therapy for PDAC may inform the clinician about tumor biology through real time assessment of tumor response to chemotherapy, address the entity of radiographically silent micrometastatic disease, and ultimately allow for the selection of the optimal surgical candidate. In clinical practice, due to high rates of recurrence and poor outcomes in PDAC there are multiple academic institutions that are proponents of using highly effective metastatic regimens such as FOLFIRINOX or gemcitabine/nab-paclitaxel for ‘borderline resectable’ and more recently resectable pancreatic cancer in the neoadjuvant setting, albeit for all comers. Although clinical trials are actively ongoing to elucidate the benefit of neoadjuvant chemotherapy in both of these settings, to date there is no clear consensus guidelines regarding a standardized neoadjuvant approach for PDAC. The use of a clinically meaningful biomarker, such as KOC expression, in order to identify high risk patients upon diagnosis may help refine which patients in fact would benefit most from a neoadjuvant versus a surgery first approach for the management of resectable PDAC. However, further large scale prospective investigation of KOC expression patterns and outcomes in PDAC are needed to validate its use as a prognostic biomarker. Considering that KOC expression is easily assessed by IHC staining on tumor tissues it can be performed in both community practices and academic settings once validated. Whether or not KOC expression is predictive for resistance or response to chemotherapeutic regimens in pancreatic cancer also needs further investigation.
Conclusions
The prognosis of pancreatic cancer remains grave with a five-year OS less than 5%. There is a desperate need for novel approaches to the management of this disease especially considering the median survival for patients who undergo curative intent surgery remains less than 24 months. Identification of a prognostic biomarker in patients with PDAC upon initial diagnosis can serve as a way to individualize and subsequently optimize treatment options. We report our results of a retrospective review of sixty-two patients who underwent curative intent surgery for PDAC in which immunohistochemical overexpression of KOC from tumor tissue represented a marker of aggressive biology reflecting early recurrence and distant metastasis. In an era where neoadjuvant chemotherapy is becoming a new paradigm for the management of borderline resectable and resectable pancreatic cancer, the identification of clinically relevant prognostic biomarkers can further enhance appropriate patient selection. Large scale prospective clinical trials are necessary to validate the prognostic utility of KOC as well as further investigate KOC overexpression and outcomes with the use of neoadjuvant chemotherapy, particularly in resectable pancreatic cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Geisinger Medical Center Institutional Review Board in Danville, Pennsylvania (approval number: 2012-0110).
References
- Estimated new cases are based on 1998-2012 incidence data reported by the North American Association of Central Cancer Registries (NAACCR). Estimated deaths are based on 1998-2012 US mortality data, National Center for Health Statistics, Centers for Disease Control and Prevention.
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Müeller-Pillasch F, Lacher U, Wallrapp C, et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997;14:2729-33. [Crossref] [PubMed]
- Yantiss RK, Woda BA, Fanger GR, et al. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol 2005;29:188-95. [Crossref] [PubMed]
- Schaeffer DF, Owen DR, Lim HJ, et al. Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) overexpression in pancreatic ductal adenocarcinoma correlates with poor survival. BMC Cancer 2010;10:59. [Crossref] [PubMed]
- Bandziulis RJ, Swanson MS, Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev 1989;3:431-7. [Crossref] [PubMed]
- Mueller F, Bommer M, Lacher U, et al. KOC is a novel molecular indicator of malignancy. Br J Cancer 2003;88:699-701. [Crossref] [PubMed]
- Sitnikova L, Mendese G, Liu Q, et al. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res 2008;14:1701-6. [Crossref] [PubMed]
- Jiang Z, Chu PG, Woda BA, et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol 2006;7:556-64. [Crossref] [PubMed]
- Hoffmann NE, Sheinin Y, Lohse CM, et al. External validation of IMP3 expression as an independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma. Cancer 2008;112:1471-9. [Crossref] [PubMed]
- Köbel M, Xu H, Bourne PA, et al. IGF2BP3 (IMP3) expression is a marker of unfavorable prognosis in ovarian carcinoma of clear cell subtype. Mod Pathol 2009;22:469-75. [Crossref] [PubMed]
- Dalerba P, Sahoo D, Paik S, et al. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. N Engl J Med 2016;374:211-22. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. Programmed death-1 blockade in mismatch repair deficient colorectal cancer. J Clin Oncol 2016;34:abstr 103.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Heinrich S, Pestalozzi B, Lesurtel M, et al. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer 2011;11:346. [Crossref] [PubMed]


