Advanced biliary tract cancer: clinical outcomes with ABC-02 regimen and analysis of prognostic factors in a tertiary care center in the United States
Introduction
Biliary tract cancers (BTC) are diverse malignancies arising from the biliary tract epithelium either intrahepatic or extrahepatic biliary tract. There are about 14,000 new cases per year in the United States (1,2). Most cases of BTC are lethal due to advanced disease at presentation or high relapse rate after local treatment (3-5). Untreated patients with advanced disease have a short survival of 3–4 months (3). Few patients who present with local disease and are potential candidates for surgical resection based on specific radiologic and clinical criteria can be treated surgically (3,6). The outcome of these patients is still dismal with a 5-year survival of 30–40 percent for intrahepatic disease (3). Defining a unified criteria for resectability for BTC is challenging as resectability may differ based on the site of disease: intrahepatic, hilar or extrahepatic cholangiocarcinoma (EHC) (7). Several other factors such as the condition of the patient, expertise of the surgeon and the hospital, and biology are crucial in this decision-making process (7). There is limited data for effective management of advanced unresectable disease. In the absence of standard therapy and randomized phase III clinical trials before 2007, Eckel et al., attempted to identify superior regimen by analyzing available data which consisted of several small and nonrandomized studies (8). This pooled analysis included 112 trial arms and 2,810 patients, and demonstrated that combination chemotherapy with gemcitabine and cisplatin or oxaliplatin increased response rates in advanced BTC’s (8). The first randomized phase II study published in 2007 showed that gemcitabine and cisplatin (GC) had a superior time to progression (8 months) compared to 4 months with Gemcitabine alone (9). Based on the results of the phase II study, the same group conducted a randomized phase III trial comparing GC with gemcitabine alone in which 410 patients were randomized to either of the two groups (4). GC provided an overall survival (OS) advantage over gemcitabine alone in the ABC-02 clinical trial (11.7 vs. 8.1 months) in locally advanced or metastatic intrahepatic cholangiocarcinoma (IHC), EHC, gallbladder cancer (GBC), or ampullary cancer (4). With the exception of neutropenia, both groups had similar adverse events (4).
Since the ABC-02 trial, GC is the most commonly used regimen for treating advanced BTC in United States (10). There are other authors who have questioned the role of combination therapy such as a Korean retrospective study that showed that gemcitabine alone might also be an option for advanced BTC (11).
There are several challenges in management of cholangiocarcinoma (3). Metastatic cholangiocarcinoma is often combined with unresectable locally advanced disease in clinical trials and registries (2,4). The results of these studies should be interpreted with caution as patients with metastatic disease have a worse survival compared to regional disease (2) as metastatic diseases may have a more aggressive biology (2,3,12). This is suggested by a genomic analysis study of cholangiocarcinoma in which it was shown EHC with mutations in BAP1 and PBRM1 are more likely to be associated with bone metastases and worse survival (13). This was also suggested in the ABC-02 trial where the benefit seen in metastatic disease (HR =0.74) was not as remarkable as in the unresectable locally advanced (HR =0.47) (4,14).
The poor prognosis associated with advanced BTC with or without treatment suggests the need for careful selection of patients who may benefit more from chemotherapy or clinical trials. Prognostic factors in advanced BTC have not been well established (5). Although previous studies have attempted to identify predictive and prognostic factors, currently there is no reliable prognostic model that can be used clinically to risk-stratify patients. Before the ABC-02 trial, a Korean study included 213 patients from 2000–2007, and identified poor performance status (PS), metastatic disease, IHC, liver metastases, and elevated alkaline phosphatase (ALP) as poor prognostic factors (15). Based on these factors the authors developed a prognostic model and stratified patients into low-risk, intermediate-risk, and high-risk groups (15). The median OS was 11.5, 7.3 and 3.6 months for the low-risk, intermediate-risk and high-risk group respectively (15). Other studies have suggested prognostic roles for blood counts, bilirubin and gender (14). Data for survival based on prognostic factors is conflicting and there is a critical need to identify clinically reliable biomarkers that could predict response and provide prognostic information allowing informed decision making (5,14-16).
Thus, the goal of our study was to analyze the outcomes of metastatic cholangiocarcinoma treated with GC regimen and to identify potential predictive and prognostic indicators of response and survival in this group of patients.
Methods
We conducted a retrospective analysis of clinical outcomes of stage IV advanced BTC treated at a single tertiary care institution. The study was approved by institutional review board (IRB) of University of Cincinnati (No. #IRB00000180) and IRB waived the requirement to obtain informed consent for all adult participants as it presented no greater than minimal risk.
Hypothesis and aims
Our hypothesis was that the OS and progression free survival (PFS) of metastatic BTC patients treated with platinum and gemcitabine combinations at University of Cincinnati are similar to the outcomes reported in ABC-02 trial. Specific aims were to study the PFS and OS in patients who received ABC-02 regimen in advanced disease setting and to identify the role of baseline tumor markers [CA19-9, carcinoembryonic antigen (CEA)], liver function tests and other clinical variables such as age, sex, stage, PS, metastasis and location of tumor in predicting response in advanced BTC patients treated with ABC-02 regimen.
Study design and participants
All adult patients, 18 years or older with advanced BTC treated between the period of January 2008 and January 2015 at University of Cincinnati that were eligible based on defined criteria were included in the analyses. For the purpose of this analysis we only included patients who had metastatic disease at presentation [stage IV (a or b)] (17) and were treated with GC as their initial chemotherapy regimen. All available medical records were accessed from electronic health records and paper charts if available. Data was retrieved using International Statistical Classification of Diseases—Ninth Edition (ICD-9) codes as necessary. Tumors were classified into intra-hepatic cholangiocarcinoma (IHC), extra-hepatic cholangiocarcinoma (EHC) and gallbladder carcinoma based on clinic progress notes, pathology and imaging studies. Perihilar tumors were included with EHC. Patients were excluded if they did not have complete records. Those patients who did not have any follow up visits after initiating chemotherapy were excluded from the study.
GC was given as per ABC-02 protocol [cisplatin (25 mg/m2) followed by gemcitabine (1,000 mg/m2) on days 1 and 8, every 21 days] (4) with appropriate modifications until disease progression or unacceptable toxicities.
Statistical analysis
OS was calculated from the date of chemotherapy initiation to date of death or censored at last follow-up. PFS was calculated from the date of chemotherapy initiation to date of progression or death which ever was earlier. Kaplan Meier method was used to calculate PFS, and OS. Cox model was used to test the association between baseline variables and OS/PFS, adjusting for gender and age at diagnosis.
For descriptive analysis numerical variables such as values of baseline tumor markers, lab values (for example—CA19-9, CEA, liver function tests) and age were summarized in median (range) and categorical or binary variables such as sex, stage, PS, and location of tumor were summarized in frequency (%). Relationships between numerical variables were assessed using spearman’s correlation coefficients. All statistical analyses were performed using SAS 9.4 software (SAS, Cary, NC, USA).
Results
A total of 26 patients met inclusion criteria: histologically proven metastatic BTC that received GC as their initial chemotherapy. In 4 of the patients (15%) carboplatin or oxaliplatin was substituted for cisplatin for tolerability.
Median age at diagnosis of the cohort was 62 years (range, 31–81 years). Most patients had IHC (69%). There were more males (61%) and more patients with PS 0–1 (69%) (Table 1).
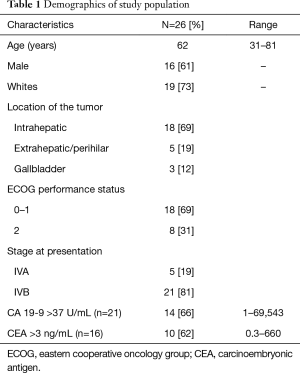
Full table
CA19-9 data was available on 21 patients (range, 1–69,543 U/mL) before starting systemic chemotherapy. Fourteen patients had abnormal (>37 U/mL) CA19-9. Follow up CA19-9 was available in 11 of these 14 patients. In the three patients for whom CA 19-9 was missing, one was lost to follow up and data was not available in the chart on other two. Eight patients out of the eleven had a drop in CA 19-9 after treatment. It decreased to >50% in 5/8 (62%) of patients.
Survival analysis
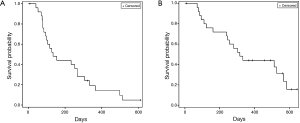
PFS of the whole cohort was 4.5 months (95% CI: 3.1–8.9 months) (Figure 1A) and OS was 10.5 months (95% CI: 7.9–18.8 months) (Figure 1B). OS at 6 and 12 months was 69% (18/26) and 42% (11/26).
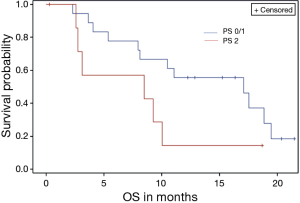
Survival analysis was also conducted based on the following baseline variable for OS and PFS: Diagnosis (IHC/EHC/GBC), PS, metastasis to liver, metastasis to lungs, and stage. None of the above variables had any significant impact on PFS. Patients with eastern cooperative oncology group (ECOG) (18) PS of 2, were noted to have a non-significant increased risk of death (P value =0.073) (Figure 2).
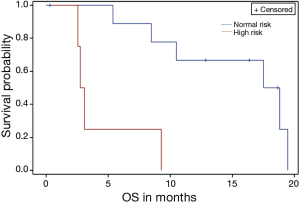
We also identified a group of high risk patients based on three risk factors (PS 2 or above, stage IVb, high CEA >3 ng/mL). In the group of patients who had all three risk factors the median survival was 2.9 months (range, 2.5–9.2 months), which was significantly worse compared to the rest of the population (median 18 months, range 5.3–19.4 months) (P<0.01) (Figure 3).
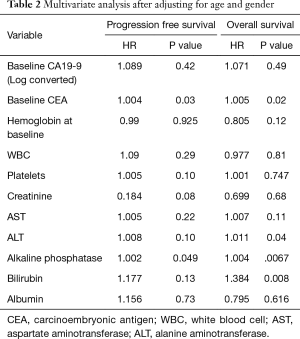
On multivariate analysis after adjusting for age and gender, increase in baseline CEA and ALP was associated with increased risk of progression (P valve <0.05) while increase in baseline CEA, alanine aminotransferase, ALP and total bilirubin was associated with increased risk of death (Table 2).
Full table
Following failure of first line treatment several of our patients received 2nd (38%) and 3rd (11%) line therapy (%). Of the 10/26 (38%) who received 2nd line chemotherapy, 9/10 received 5FU based chemotherapy. Eleven percent (3/26) received 3rd line chemotherapy.
Discussion
The OS seen in our analysis was 10.5 months and appears comparable to the median OS results seen in ABC-02 trial. In the ABC-02 trial, which established GC as a standard of care in patients with locally advanced unresectable and metastatic BTC, the median OS was 11.7 months and PFS was 8 months in the GC arm compared to 8.1 and 5 months in the gemcitabine only arm (4). Differences seen could be explained by the fact that all our patients had metastatic disease and 31% patients had an ECOG PS of 2 compared to 74% metastatic disease and 12% with ECOG PS of 2 in the ABC-02 trial (4). In the ABC-02 trial, patients with locally advanced disease were analyzed together with metastatic disease for survival analyses (4). Combining metastatic disease with locally advanced disease may complicate the interpretation, as the outcomes of metastatic disease may be different from locally advanced disease (2,4). As per the SEER database for liver and intrahepatic biliary ductal cancers, the five year survival for distant metastatic disease is 3% compared to 11% for regional disease (2). In the ABC-02 trial patients with metastatic disease had relatively less benefit [hazard ratio (HR) for death 0.74 (0.57–0.95)] compared to patients with locally advanced disease [HR 0.47 (0.29–0.74)] (4). In our analysis we focused on metastatic disease only and have identified factors in this selective group that could be of predictive and prognostic value.
Assessment of clinical efficacy from clinical trials of cholangiocarcinoma is complicated as most studies combine IHC, EHC, GBC and occasionally ampullary carcinomas (3,4,14). Locally advanced disease is also often combined with metastatic disease.(4) Previous studies have identified the role of site of diseases and metastasis in prognosis of BTC treated with GC (4,5,14-16). Liver function tests such as ALP and bilirubin have also been included in prognostic models (5,14,15). In our study, we also identified the role of baseline liver function tests in predicting response. Increase in baseline alanine aminotransferase, ALP and total bilirubin was associated with increased risk of death while increase in ALP was associated with increased risk of progression. Additionally, we also identified a potential role of CEA as prognostic biomarker. Tumor markers: CA 19-9 and CEA have well established role in management of pancreatico-biliary cancer (6). Tumor markers are often monitored during the course of treatment in metastatic setting as a potential indicator for response. The role of tumor markers as prognostic biomarkers has been studied before in pancreatic and BTC. CA 19-9 have been well studied as a predictor of response in pancreatic cancer patients (19), however similar evidence does not exist for BTC. In our study, increase in pretreatment CEA was found to have an increased risk of progression and death, while pretreatment CA 19-9 was not associated with survival. Previous studies have also suggested the role of hemoglobin, white blood cell count and absolute neutrophils count as prognostic indicators (14). In our analysis we did not encounter similar findings. Larger prospective studies are needed to further establish the role of baseline tumor markers and liver function tests. From our study results, we do recommend considering checking CEA and liver function tests at baseline and during the course of therapy with cisplatin and gemcitabine.
Our study had a 31% ECOG PS-2 patients and we identified a trend towards increased risk of death in these patients (P value =0.073). The ABC-02 trial had only 12 percent patients with PS 02 or above which raises questions about the efficacy of GC in PS-02 patients. Single agent therapy with gemcitabine, capecitabine or combination of gemcitabine and oxaliplatin may be reasonable options for PS-02 patients who may not tolerate GC (20,21). In our study population we found that cisplatin was substituted with oxaliplatin or carboplatin in 4 (15%) patients for better tolerability. There are no randomized studies specifically looking at first line systemic chemotherapy in cholangiocarcinoma patients with borderline PS. The GERCOR study published in 2004 prospectively used gemcitabine and oxaliplatin (GEMOX) in advanced cholangiocarcinoma patients (20). They divided their patients into group A, who had PS 0–2, bilirubin <2.5× normal, received GEMOX as first-line chemotherapy and group B, who had PS >2 and/or bilirubin >2.5× normal and/or prior chemotherapy. In group B (n=23) which had patients with poor PS the PFS was 3.9 months and OS 7.6 months (20). The results of this study suggested that GEMOX could be a reasonable option in patients with poor PS or patients who are not candidates for cisplatin.
Poor ECOG PS is the most consistent indicator in most studies attempting to identify prognostic markers in advanced BTC (5,14,15,22). Poor PS and other factors have been included in prognostic models previously (15). In our study, a group of patients with three high risk features (ECOG PS-2 or above, stage IVb and high CEA >3 ng/mL) did significantly worse with median survival of 2.9 months (range, 2.5–9.2 months) compared to the rest of the population. We chose to combine these three factors because metastatic disease and poor performance has previously been associated with poor survival (14). In addition, our study showed that increased CEA is associated with increased risk of death. Although the components of the prognostic models are different, the survival for high-risk group in our study was dismal similar to the data previously reported in other prognostic models (5,14,15). We recommend that caution should be exercised when considering this group of patients for systemic chemotherapy and future clinical trials may want to keep these factors in mind.
Thirty eight percent of our patients received 2nd line and 11% received 3rd line therapy. Nine of the ten patients who received 2nd line regimen received 5FU based chemotherapy. Approximately 30–40% advanced BTC patients who get gemcitabine-based treatment in first line, get second line treatment that is most often 5-flurouracil based (23,24). There are no published randomized phase 3 clinical trials for cholangiocarcinoma in the second line setting and the optimal candidates and regimen for second-line chemotherapy is not well defined. Based on data from a large retrospective study, the median survival for patients getting second line treatment is approximately 6 months (24). ECOG PS, CA 19-9, response to first line therapy and previous surgery have been identified as prognostic factors in second line treatment in advanced BTC (25).
Due to the overall poor prognosis of metastatic cholangiocarcinoma and limited treatment options, there is a clear need for identification of novel treatment options (26-29). The role of anti-angiogenics and other targeted therapies have been evaluated in first and second line treatment of cholangiocarcinoma either in combination with gemcitabine based chemotherapy or 5 fluorouracil based therapy (26,27,29-31). In a phase 2 study, GEMOX with bevacizumab (GEMOX-B) showed tolerable safety in patients with advanced BTCs (26). GEMOX-B was given to 35 patients with advanced BTCs, 32 of which had not received any systemic therapy before. Median PFS was 7.0 months (95% CI: 5.3–10.3 months) and OS was 12.7 months (95% CI: 7.3–18.1 months) in the whole group and 7.6 months (95% CI: 5.9–12.4 months) and 14.2 months (95% CI 6.8–22.0 months) in patients with IHC (26). Whether these results are better than can be achieved with GEMOX alone or GC will require a randomized clinical trial. Agents targeting epidermal growth factor receptor (EGFR) have also been evaluated in cholangiocarcinoma patients (30-32). Cetuximab, an anti-EGFR monoclonal antibody showed some response in a single arm phase II study but the benefits were not found to be significant in a randomized clinical trial (30,32). In the randomized phase II study, 76 patients were assigned to GEMOX plus cetuximab and 74 to chemotherapy alone. Median PFS was 6.1 months (95% CI: 5.1–7.6 months) and OS was 11.0 months (95% CI: 9.1–13.7 months) in the chemotherapy plus cetuximab group compared to 5.5 months (95% CI: 3.7–6.6 months) and 12.4 months (95% CI: 8.6–16.0 months) in the chemotherapy alone arm (32). Erlotinib which is a tyrosine kinase inhibitor targeting the EGFR pathway has also shown some clinical activity (31). GEMOX was combined with erlotinib in a Korean multicenter, open-label, phase 3 randomized trial (31). Two hundred sixty-eight patients with previously untreated advanced cholangiocarcinoma, gallbladder cancer (GBC), or ampullary cancer were assigned to GEMOX with (n=135) or without (n=133) erlotinib (100 mg daily) (31). In the subgroup with cholangiocarcinoma (n=180), the median PFS in GEMOX plus erlotinib was 5.9 months compared to 3 months in GEMOX only group and was statistically significant (31). Although the GEMOX with erlotinib regimen looks promising, comparison with GC in a randomized powered study is warranted before it could be considered a standard treatment option. Immunotherapy alone or in combination with targeted therapy or cytotoxic chemotherapy in BTC could also have therapeutic potential in cholangiocarcinoma and is an area under active investigation (33).
Our study is limited with regards to its conclusion since it is a retrospective, single institutional study with a small patient population. We decided to only include patients with metastatic disease in this analysis that is different from previous studies that have combined metastatic and locally advanced disease together (4,11,14,15). Through our study we confirmed the findings of the ABC-02 trial in patients with metastatic cholangiocarcinoma and have suggested a simple prognostic model that can identify poor risk patients.
In conclusion, GC is an effective regimen in patients with metastatic BTC. Careful use of prognostic factors such as PS, stage IVb and elevated CEA (>3 ng/mL) may risk stratify patients and assist in clinical decision making. Further large scale prospective studies are warranted to validate our findings and carefully select patients who may or may not benefit from systemic chemotherapy.
Acknowledgements
The authors thank all our patients and their families.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board (IRB) of University of Cincinnati (No. #IRB00000180) and IRB waived the requirement to obtain informed consent for all adult participants as it presented no greater than minimal risk.
References
- Ciombor KK, Goff LW. Current therapy and future directions in biliary tract malignancies. Curr Treat Options Oncol 2013;14:337-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189-200. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Peixoto RD, Renouf D, Lim H. A population based analysis of prognostic factors in advanced biliary tract cancer. J Gastrointest Oncol 2014;5:428-32. [PubMed]
- Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9:43-57. [Crossref] [PubMed]
- Schulick RD. Criteria of unresectability and the decision-making process. HPB (Oxford) 2008;10:122-5. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [Crossref] [PubMed]
- Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer 2009;101:621-7. [Crossref] [PubMed]
- Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: An update. World J Gastrointest Oncol 2013;5:171-6. [Crossref] [PubMed]
- Hwang IG, Song HS, Lee MA, et al. Treatment outcomes of gemcitabine alone versus gemcitabine plus platinum for advanced biliary tract cancer: a Korean Cancer Study Group retrospective analysis. Cancer Chemother Pharmacol 2014;74:1291-6. [Crossref] [PubMed]
- Brandi G, Farioli A, Astolfi A, et al. Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies. Oncotarget 2015;6:14744-53. [Crossref] [PubMed]
- Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 2014;9:e115383. [Crossref] [PubMed]
- Bridgewater J, Lopes A, Wasan H, et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann Oncol 2016;27:134-40. [Crossref] [PubMed]
- Park I, Lee JL, Ryu MH, et al. Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first-line palliative chemotherapy. Cancer 2009;115:4148-55. [Crossref] [PubMed]
- Park JO, Oh DY, Hsu C, et al. Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review. Cancer Res Treat 2015;47:343-61. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. editors. AJCC cancer staging manual (7th ed). New York, NY: Springer, 2010.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Heinemann V, Schermuly MM, Stieber P, et al. CA19-9: a pedictor of response in pancreatic cancer treated with gemcitabine and cisplatin. Anticancer Res 1999;19:2433-5. [PubMed]
- André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [Crossref] [PubMed]
- Patt YZ, Hassan MM, Aguayo A, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 2004;101:578-86. [Crossref] [PubMed]
- Suzuki E, Furuse J, Ikeda M, et al. Treatment efficacy/safety and prognostic factors in patients with advanced biliary tract cancer receiving gemcitabine monotherapy: an analysis of 100 cases. Oncology 2010;79:39-45. [Crossref] [PubMed]
- Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 2013;49:329-35. [Crossref] [PubMed]
- Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 2015;121:3290-7. [Crossref] [PubMed]
- Fornaro L, Cereda S, Aprile G, et al. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer 2014;110:2165-9. [Crossref] [PubMed]
- Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 2010;11:48-54. [Crossref] [PubMed]
- Guion-Dusserre JF, Lorgis V, Vincent J, et al. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J Gastroenterol 2015;21:2096-101. [PubMed]
- Subbiah IM, Subbiah V, Tsimberidou AM, et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: eliciting early response signals from phase 1 trials. Oncotarget 2013;4:156-65. [PubMed]
- Marks EI, Yee NS. Molecular genetics and targeted therapeutics in biliary tract carcinoma. World J Gastroenterol 2016;22:1335-47. [Crossref] [PubMed]
- Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol 2010;11:1142-8. [Crossref] [PubMed]
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181-8. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Marks EI, Yee NS. Immunotherapeutic approaches in biliary tract carcinoma: Current status and emerging strategies. World J Gastrointest Oncol 2015;7:338-46. [Crossref] [PubMed]


