Disparities in receipt of radiotherapy and survival by age, sex, and race among patients with non-metastatic squamous cell carcinoma of the anus
Introduction
Squamous cell carcinoma of the anus (SCCA) constitutes 2.4% of gastrointestinal cancers. An estimated 7,210 new cases and 950 deaths in the United States are predicted for 2014 (1). Both the incidence and mortality of anal cancer has been increasing in the U.S. with an annual percentage change of 1.9% and 3.6%, respectively (1).
Current standard of care for patients with non-metastatic SCCA is chemoradiotherapy with mitomycin and 5-fluorouracil (1). This is based on findings of the EORTC trial that investigated the addition of mitomycin/5-fluorouracil to the traditional radiotherapy (RT) regimen and found an increased rate of complete remission (80% vs. 54%) (2). Ajani et al. (3) investigated the replacement of mitomycin with cisplatin, but found no improvement in disease-free survival and higher rates of colostomy in the cisplatin group.
Blacks have higher annual percentage increases in incidence and mortality of SCCA compared to whites (2.3 vs. 2.0 and 4.4 vs. 3.6, respectively) (1). Furthermore, blacks with SCCA suffer from lower survival rates compared to whites [5-year relative survival (RS) 56% vs. 67%]. It is unclear whether the inferior survival rates are due to disparities in cancer care in these patients. Thus, we conducted this study to evaluate disparities in receipt of RT and survival in patients with non-metastatic squamous cell carcinoma.
Methods
Data source
We used the Surveillance, Epidemiology, and End Results (SEER) program database to identify patients with SCCA diagnosed between 1998 and 2008 (4). The SEER 18 database is a population-based cancer database sponsored by the National Cancer Institute that collects high-quality incidence and survival data from several cancer registries throughout the United States (4). The SEER program’s standard for case ascertainment is 98%, and this goal is achieved by conducting rigorous quality control studies every other year (5). The SEER database covers approximately 28 percent of the US population overall and 26 percent of African Americans (4). We gathered data using registries from the following areas: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, greater California, Kentucky, Louisiana, New Jersey, and greater Georgia.
Study population
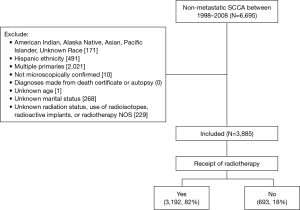
We identified a total of 6,695 patients with non-metastatic (localized or regional) carcinoma of the anus diagnosed between January 1, 1998 and December 31, 2008. We defined SCCA as cases with ICD-O-3 histology codes 8051 through 8081. Inclusion criteria were non-Hispanic black or white race and no prior malignancies. American Indian/Alaska Native and Asian/Pacific Islander patients were excluded because of insufficient numbers for meaningful analysis. Patients were excluded if their disease was not histologically confirmed, their diagnosis was made from death certificate or autopsy report, or they had missing data for age, marital status, or RT status. Patients receiving radioactive implants, radioisotopes, or with unspecified mode of radiation were also excluded. The final study population was N=3,885 (Figure 1).
Study variables
We assessed the variables age, sex, race, stage, marital status, and RT status. We dichotomized age as <65 or 65+ years old. Marital status separated patients into three groups; married (including common law), single (never married), and S/D/W/U (separated, divorced, widowed, unmarried or domestic partner). Stage was either localized or regional based on the LRD stage variable in SEER (6). We divided treatment groups into those who received RT (beam radiation only) and those who did not (including those who refused RT).
Statistical analysis
We used univariate logistic regression to assess the relationship between independent variables and receipt of RT and reported unadjusted odds ratios (ORs). To adjust for covariates we used a multivariate logistic regression model and reported adjusted ORs. These statistical analyses were performed using Stata 13.0 (StataCorp, College Station, TX, USA).
We calculated 1- and 5-year RS rates using survival sessions in the SEER*Stat program (7). RS is a population-based measure of net-survival calculated as a ratio of the proportion of observed survivors (all causes of death) in our study population to the proportion of expected survivors in a group of individuals matched by age, sex, race, and year. The use of RS is based on the assumption that death from anal cancer is a negligible proportion of deaths in the general population, a reasonable assumption for a relatively rare cancer such as SCCA (8). We calculated hazard ratios (HRs) while adjusting for each covariate using Cox proportional hazards model in the CanSurv software package (9).
Results
Patient characteristics and factors associated with receipt of RT
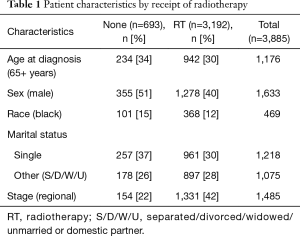
A total of 3,885 patients (Figure 1) were identified with SCCA as the first primary cancer that met our inclusion criteria. The median age at diagnosis was 56 years old, 30% were 65+ years old [1,176], 42% were male [1,633], 12% were black [469], and 38% had regional disease at the time of diagnosis [1,485]. See Table 1 for details.
Full table
Factors associated with receipt of RT
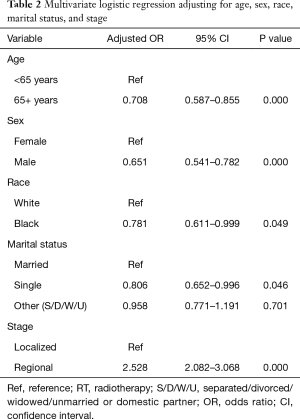
Of the patients studied, 3,192 (82%) received RT. A significant racial difference existed between whites and blacks with 83% of whites and 78% of blacks receiving RT. After adjusting for covariates age, sex, marital status, and stage, black patients were less likely to receive RT compared to whites (adjusted OR 0.781; 95% CI: 0.611–0.999; P=0.049). Older patients (65+ years) were less likely to receive RT compared to younger patients: 80% vs. 83% (adjusted OR 0.71; 95% CI: 0.59–0.86; P<0.001), and females were more likely to receive RT compared to males: 85% vs. 78% (adjusted OR 1.54; 95% CI: 1.28–1.85; P<0.001). Single patients and those with localized disease were also less likely to receive RT compared to their referent groups. See Table 2 for details.
Full table
Factors associated with RS
Among patients receiving RT, black patients had worse RS at 1 and 5 years compared to whites (1-year RS 88.4±1.8 vs. 93.0±0.5, P<0.005; 5-year RS 65.7±2.8 vs. 75.8±1.0, P<0.001). Older patients had worse RS than those <65 years old (1-year RS 88.0±1.2 vs. 94.3±0.5, P<0.001; 5-year RS 70.3±2.1 vs. 76.4±1.0, P<0.005). Males had worse RS at 1 and 5 years compared to females (1-year RS 90.0±0.9 vs. 94.2±0.6, P<0.001; 5-year RS 68.8±1.5 vs. 78.6±1.2, P<0.001).
Factors associated with survival: Cox model analysis
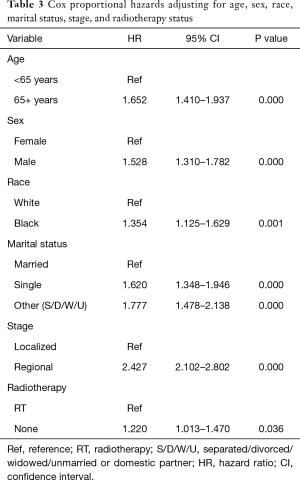
In order to assess RS while adjusting for covariates, Cox proportional hazards model was used. In this analysis, blacks had worse RS compared to whites (adjusted HR 1.35; 95% CI: 1.13–1.63; P=0.001). Older patients had worse RS compared to those <65 years old (adjusted HR 1.65; 95% CI: 1.41–1.94; P<0.001), and males had worse RS compared to males (adjusted HR 01.53; 95% CI: 1.31–1.78; P<0.001). See Table 3 for details.
Full table
Discussion
Receipt of RT
Since the release of the Institute of Medicine’s landmark report, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, increased attention has been directed toward healthcare disparities in the United States (10). Much of this attention has been aimed at disparities affecting blacks (11). Lower treatment rates are found among black patients for various cancers (11-14). These disparities may arise from a number of sources including lower SES (10). In the majority of studies that controlled for SES, disparities in treatment significantly diminished after adjustment, but persisted nonetheless (10). This makes it likely that, in addition to SES, sociocultural factors or provider biases are playing a role in their lower rates of treatment.
Additionally, older patients and men received lower rates of RT compared to younger patients and females. This is consistent with a study by Asch et al that assessed receipt of care according to age and sex and found that older patients (52% vs. 58%) and males (52% vs. 57%) were both less likely to receive recommended care (15). While older patients are eligible for Medicare and may see fewer access related barriers, they also have greater incidence of potentially RT-limiting comorbidities such as anemia (16). Age-related disparities in treatment of other cancers have been documented (17,18).
Survival
Our finding of worse RS among blacks matches the disparity noted in a recent study where blacks had a lower 5-year RS rate compared to whites (56% vs. 67%) (1). Although we did not see a 5-year survival difference in our cohort, our multivariate analysis showed greater risk of death among blacks (adjusted HR 1.35; P=0.001). This same analysis found a greater risk of death among those not receiving RT (adjusted HR 1.22; P=0.036), and may be a reason black patients had worse survival.
The men in our study also had worse survival. Bartelink et al. identified male sex as a negative prognostic factor in SCCA (2). This poorer prognosis may be due to higher incidence of HIV/AIDS among males and their subsequent increased risk of developing SCCA (19). Thus, male sex may act as a proxy for HIV status, an important comorbidity leading to lower RS in that group.
Our study has several limitations. One of the major limitations is the lack of detail regarding therapeutic interventions such as dosage, regimens, missed appointments, and delays in initiation of treatment after diagnosis. Also, SEER does not collect data on comorbidities, socioeconomic status (except at the county level), or data regarding important risk factors such as HIV infection, HPV infection, and payer status.
In conclusion, there are significant disparities in the receipt of RT and survival in patients with non-metastatic SCCA. Identification of factors responsible for differences in treatment may lead to interventions to reduce disparities and improve SCCA outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: No formal approval of IRB is required as data were collected from a source that was publicly available and did not contain unique patient identifiers. Informed consent is not required as it was a SEER database study and data could not be tracked back to individuals.
References
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, Available online: http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [PubMed]
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914-21. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program () SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2013 Sub (1973-2011 varying) - Linked To County Attributes- Total U.S., 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014 (updated 5/7/2014), based on the November 2013 submission.www.seer.cancer.gov
- Surveillance, epidemiology and end result [Internet]. Bethesda, MD: National Cancer Institute, Surveillance Systems Branch; 24 April 2013. Available online: http://seer.cancer.gov/data/metadata.html#2
- Young JL Jr, Roffers SD, Ries LA, et al. editors. SEER Summary Staging Manual - 2000: Codes and Coding Instructions, National Cancer Institute, NIH Pub. No. 01-4969, Bethesda, MD, 2001.
- Surveillance Research Program, National Cancer Institute SEER*Stat software () version 8.1.5.seer.cancer.gov/seerstat
- Brown CC. The statistical comparison of relative survival rates. Biometrics 1983;39:941-8. [Crossref] [PubMed]
- Cansurv, Version 1.3. February 2014; Statistical Research and Applications Branch, Data Modeling Branch, National Cancer Institute.
- Smedley BD, Stith AY, Nelson AR et al. Unequal treatment: confronting racial and ethnic disparities in health care. National Academies Press, 2009.
- Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 2002;94:334-57. [Crossref] [PubMed]
- Howell EA, Chen YT, Concato J. Differences in cervical cancer mortality among black and white women. Obstet Gynecol 1999;94:509-15. [PubMed]
- Cooper GS, Yuan Z, Landefeld CS, et al. Surgery for colorectal cancer: Race-related differences in rates and survival among Medicare beneficiaries. Am J Public Health 1996;86:582-6. [Crossref] [PubMed]
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341:1198-205. [Crossref] [PubMed]
- Asch SM, Kerr EA, Keesey J, et al. Who is at greatest risk for receiving poor-quality health care? N Engl J Med 2006;354:1147-56. [Crossref] [PubMed]
- Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer 1997;80:1273-83. [Crossref] [PubMed]
- Chagpar R, Xing Y, Chiang YJ, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol 2012;30:972-9. [Crossref] [PubMed]
- Patel HD, Kates M, Pierorazio PM, et al. Race and sex disparities in the treatment of older patients with T1a renal cell carcinoma: a comorbidity-controlled competing-risks model. Urol Oncol 2014;32:576-83. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Rock CE, et al. Outcomes and prognostic factors for squamous-cell carcinoma of the anal canal: analysis of patients from the National Cancer Data Base. Dis Colon Rectum 2009;52:624-31. [Crossref] [PubMed]