Gastrointestinal stromal tumors (GISTs): point mutations matter in management, a review
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the intestinal track and are thought to originate from the interstitial cells of Cajal (ICCs). There are approximately 5,000 new cases diagnosed each year and an ever-growing prevalence (1). While GISTs can arise anywhere from esophagus to rectum, the majority originate in the stomach (1,2). Previously, GISTs were very difficult to treat as they are refractory to traditional cytotoxic chemotherapy (3). In the past 10 years, the rapid evolution of this field has led to the continued need to collaborate between academic and private practitioners to optimize individualized therapy.
Management of GIST patients is divided into resectable disease that may need neoadjuvant/adjuvant therapy and metastatic disease that requires palliative tyrosine kinase inhibition. Our understanding of which therapies are most efficacious with each mutation has also established GIST as a model for targeted therapies in solid tumors. Clear guidelines have been developed regarding staging, prognosis and treatment (4). In addition, detailed provides correlations between current point mutations and response are available (https://www.mycancergenome.org/content/disease/gist/correlates). A deeper understanding of the molecular pathogenesis and driving role of the proto-oncogenes KIT and PDGRFα has transformed the treatment of both localized and metastatic disease. Herein we will review how therapeutic tyrosine kinase inhibitor (TKI) management is affected by mutational status.
Diagnosis and standard management
The diagnosis of GIST is based on a combination of imaging findings and pathology (4). Asymptomatic lesions are usually identified by upper endoscopy or as an incidental finding on other imaging modalities (5). Symptomatic GISTs are either found on imaging workup for the presenting symptom or found because of an emergent surgery in extreme cases (6). Obtaining tissue by biopsy or surgery then results in a definitive diagnosis. Historically, GISTs were considered to be of smooth muscle origin and were originally named gastric leiomyosarcomas. However, in the 1980’s electron microscopy and immunohistochemistry were used by Mazur and colleagues to demonstrate that these tumors differ from leiomyosarcomas, and as such they proposed the term GISTs (7). In the 1990’s researchers identified similarities between GISTs and ICCs, a population of cells in the gut. These cells were found to express cKIT/CD117 (8). Shortly thereafter, Hirota and colleagues discovered that GISTs express CD117 as well (2). Today, it is hypothesized that ICCs are the cell of origin for GIST and it is clear that 95% of GISTs express cKIT/CD117 by immunohistochemistry forming the basis for diagnosis of this tumor. Later in the 1990’s DOG-1 was identified as another immunohistochemical marker of GIST as it is expressed in approximately 98% of GIST (9). The combination of cKIT/CD117 and DOG-1 staining is virtually diagnostic of this tumor.
The first report of gain-of-function mutations in cKIT/CD117 in GIST paved the way for the molecular characterization of these tumors. Today, we know that 75–80% of GISTs will harbor a mutation in cKIT. It was subsequently discovered that 10% of GISTs would have a mutation in PDGFRα, leaving 10–15% of GISTs that lack a mutation in either of these genes. This group has historically been termed wild-type (WT) GIST, but this grouping is likely too broad and non-specific, as it encompasses a diverse molecular population (10). The molecular subtype of GIST (mutational characterization) is important for prognosis and treatment planning (11,12). Below we detail the clinical significance of known molecular alterations, supporting the need for sequencing all GISTs for mutations in KIT and PDGFRα at the time of diagnosis.
Adjuvant therapy data for imatinib
A role for adjuvant imatinib was first demonstrated in the ACOSOG Z9001 trial in which patients with a completely excised GIST measuring at least 3 centimeters were randomized to 1 year of imatinib versus placebo (13). While relapse free survival was improved at one year in the imatinib arm (98% vs. 83%: HR =0.35), the rate of recurrence increased in the imatinib arm approximately 6 months after completion of adjuvant therapy. Given these findings, a longer duration of therapy was examined. The Swedish study, SSG XVIII, compared 1 vs. 3 years of adjuvant imatinib in high risk cKIT positive GISTs. In this study, high-risk patients as defined as those with tumors greater than 10 cm in size, mitotic count greater than 10 mitoses/50 high power fields, 5 cm in size with a mitotic count of greater than 5 mitoses/50 high power fields, or tumor rupture at time of surgery were enrolled. In this high risk group, those patients on 3 years of adjuvant imatinib demonstrated improved relapse free survival at 5 years (65.6% vs. 47.9%), as well as an improved overall survival at 5 years (92% vs. 81.7%) (14,15). These findings have solidified the use of 3 years of adjuvant imatinib as a current standard of care for high risk resected GISTs.
TKI pathway in the metastatic setting
A TKI treatment paradigm was initially developed and has been used irrespective of point mutational status. This pathway is now evolving with current mutational understating. Imatinib given at 400 mg per day was granted accelerated FDA approval for advanced or metastatic GIST in 2002 (16). Dose escalation from 400 mg daily to 800mg (400 mg twice a day) at the time of progression has been shown to be effective at achieving prolonged disease stability (17,18). Sunitinib on 6 week cycles of 4 weeks of 50mg daily followed by 2 weeks off was subsequently approved in 2006 for imatinib-refractory patients (19). Finally, regorafenib was approved in 2013 on the basis of improved progression free survival compared to placebo in patients refractory to or intolerant of sunitinib. The approved dose for regorafenib is 160 mg daily on a 3 weeks on 1 week off schedule (20).
cKIT mutations
Over 95% of GISTs express cKIT, a cell surface transmembrane receptor tyrosine kinase. It has been nearly twenty years since Hirota’s group first identified gain of function mutations in KIT as a driver in the oncogenesis of GISTs (2). The most common mutations arise in the juxtamembrane domain encoded by exon 11, followed by mutations in the extracellular domain often due to duplications in exon 9, mutations in exons 13 and 14 which affect the ATP-binding pocket, and mutations in exon 17 which are located in the true kinase domain (Figure 1).
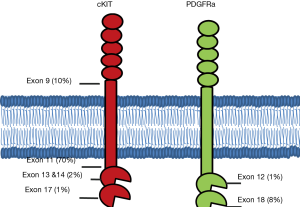
Exon 11
KIT mutations in exon 11 are the most common mutation in GISTs and are identified in approximately 70% of cases. As a group, exon 11 mutations portend a high risk of relapse after surgical resection. While the ACOSOG Z9001 trial demonstrated improved relapse free survival (HR =0.35) in patients with resected GIST when treated with a year of adjuvant imatinib compared to placebo (13), subsequent molecular profiling of tumor samples demonstrated significant heterogeneity in the benefit based on the underlying mutation. Fortunately, GIST tumors with exon 11 mutations seem to drive this benefit with a two-year relapse free survival of 91% in the imatinib arm compared to 65% in the placebo arm (P<0.0001) (21). Subsequently, the SSG XVIII trial evaluated the potential benefit of extending adjuvant therapy from 1 to 3 years (14). Exon 11 mutations demonstrate a clear benefit to extended adjuvant therapy (HR =0.31; 95% CI: 0.22–0.56). Based on these results, standard of care is to offer at least three years of adjuvant imatinib to high risk resected GISTs harboring an exon 11 mutation.
In the metastatic setting, exon 11 mutations confer an exquisite sensitivity to imatinib. Based on phase II trial data, partial response rates exceed 80% in patients with exon 11 mutations treated with 400 mg daily of imatinib, a significantly higher response rate than had been seen in any other subgroup of GIST patients (22). In addition, these responses are prolonged with a median event free survival of just under 2 years (687 days) (22). Subsequent phase III trials have failed to show any benefit to initiating higher doses of imatinib in the upfront setting for exon 11 mutations (11). In the second line setting, the benefit of sunitinib is much more modest, providing, on average, a PFS of 5.1 months (23). The benefit of third line regorafenib compared to placebo was likewise modest, but real, in this population (HR =0.212; 95% CI: 0.098–0.458) (20).
Exon 9
Nucleotide duplications in exon 9 represent the second most common mutation in GISTs and account for 10–15% of newly diagnosed cases (22). These mutations are seen much more commonly in GISTs arising from the small intestine and are relatively uncommon in those which arise in the stomach or large intestine. Compared to the more common exon 11 mutations, GISTs with exon 9 mutations tend to have better relapse free survival after curative resection. However, the exon 9 mutated GISTs benefit less from imatinib in both the adjuvant and metastatic setting. Analysis of the ACOSOG Z9001 data failed to demonstrate a statistically significant benefit in relapse free survival with 1 year of adjuvant imatinib compared to placebo (21). While this could be attributed to small sample size, the SSG XVIII trial likewise failed to show a significant benefit of 3 years of adjuvant imatinib over 1 year of treatment in the exon 9 mutated population. Further, there is no data for higher doses of imatinib in the adjuvant setting.
In the metastatic or unresectable setting, one can anticipate intermediate responsiveness to imatinib therapy. In phase II studies, partial responses were seen in 47.8% of patients treated with 400mg of imatinib (22). Two subsequent phase III studies have demonstrated that carriers of the exon 9 mutations have a better response rate to a higher dose of imatinib (800 mg daily compared to 400 mg). The progression free survival (PFS) data, however, are somewhat conflicting. In an EORTC trial, patients with exon 9 mutations had a marked improvement in PFS with a 61% relative risk reduction (P=0.0013) in disease progression, leading the authors to conclude that patients with metastatic GIST harboring an exon 9 mutation should be treated with 800mg of imatinib upfront (12). However, while the North American trial demonstrated a numerical improvement in PFS with 800mg daily (18.0 vs. 9.4 months for 800 vs. 400 mg), this finding was not statistically significant (11). Neither trial demonstrated an overall survival benefit, but crossover from 400 to 800 mg at the time of progression was allowed in each study, potentially confounding this finding.
Despite the somewhat disappointing benefits of front line imatinib, the clinical benefit of second line sunitinib can be quite impressive in the treatment of metastatic GIST with an exon 9 mutation. Heinrich and colleagues reported PFS of 19 months and overall survival over 2 years in this setting (24). Regorafenib as a third line option has demonstrated some improvement in exon 9 mutated GISTs with benefits similar to that seen in exon 11 mutated tumors (20).
Exons 13/14
Primary mutations in exons 13 and 14 of KIT are uncommon and have been identified in approximately 1–2% of newly diagnosed GISTs. Of the 397 KIT positive GISTs in the CALGB 150105 trial, 5 were found to have primary exon 13 mutations and none with exon 14. Clinical benefit to imatinib was demonstrated in 3 of these 5 patients (11).
These mutations do appear more frequently and are of greater clinical significance in the setting of secondary imatinib resistance (23). Mutations in exons 13 and 14 affect the ATP binding site, which is also the binding site for imatinib and other TKIs. Among the most common of these mutations are the V654A mutation in exon 13 and the T670I mutation seen in exon 14 (24). Sunitinib effectively inhibits these mutant kinases (25). which has translated into a clinical benefit, as sunitinib has demonstrated a significantly longer PFS for patients harboring secondary mutations in exons 13 and 14 compared to other secondary mutations, such as those in exon 17 (PFS 7.8 vs. 2.3 months; P=0.0157) (23). There is a paucity of data on the benefits of regorafenib in the low incidence mutations, and the clinical utility is unclear in this patient population.
Exon 17
Primary mutations in exon 17 are rare, accounting for approximately 1% of newly diagnosed GISTs and classically involve codons 816, 820, or 823 (26). These exon 17 mutations arise more commonly in tumors in the small intestine compared to those in the stomach or other sections of the GI tract (27). Treatment of these tumors is guided by small numbers of patients included in clinical trials. For example, 3 of 4 patients with unresectable disease with primary exon 17 mutations demonstrated clinical benefit (3 partial responses, 1 stable disease) to treatment with imatinib (12,22,). However, as secondary mutations, exon 17 mutations are often responsible for acquired imatinib resistance, potentially accounting for as many as 50% of the acquired resistance cases (28). The D816V and N822V substitutions, for example, have been associated with imatinib resistance and also confer resistance to second line sunitinib (23). Ponatinib has demonstrated strong activity against exon 17 mutations in vitro, and early studies suggest some benefit in heavily pre-treated GIST patients (28). There is new potent inhibitor of these resistance mutations, BLU-285, which is in early phases of development and has demonstrated activity in cellular assays. This suggests that a new agent that may work in imatinib driven resistance mutations (29). The development of BLU-285 has the potential to be transformative to the field of GIST therapy.
PDGFRα mutations
Mutations in PDGFRα are much less frequent than KIT mutations, and as a group, are found only in 5–10% of newly diagnosed GISTs. Gain-of-function mutations in PDGFRα, however, drive the same pathways seen in KIT mutant GISTs, namely MAP kinase and PI3K/AKT. Gain-of-function mutations have been identified in exon 18, the tyrosine kinase domain, as well exon 12, the juxtamembrane domain.
Exon 18/ D842V
Among the PDGFRα variants, mutations in exon 18 are the most common. While over a dozen specific mutations in exon 18 have been identified, the D842V substitution is the most frequent and accounts for over 60% of the PDGFRα mutations (30). In vitro studies have demonstrated that the D842V mutation confers resistance to imatinib by blocking its ability to bind to the ATP-binding site. None of the three patients with the D842V mutation in Heinrich’s analysis achieved clinical benefit from palliative imatinib therapy. In spite of limited clinical evidence, it is felt that the D842V mutation also confers resistance to sunitinib and regorafenib (22,23). However, ongoing research has demonstrated areas of promise. Ponatinib has potential activity against D842V (31). Additionally, Crenolanib is an oral TKI, which targets PDGFRAα/β and has demonstrated activity against D842V mutant GIST cell lines (as well as other imatinib resistant PDGFRα mutants) (32). Phase I-II trials have been completed, and crenolanib is moving forward in phase III trials in both Europe and the United States. In addition, BLU-285 also has demonstrated activity in D842V models and is currently in phase I testing (29).
Within non D842V exon 18 mutations, imatinib has shown significant activity in vitro. While too few of these patients have been included in clinical trials to draw a conclusion regarding the benefit of imatinib in vivo, it is felt that treatment with available TKIs is warranted. In the adjuvant setting, there was no clear benefit to three years of imatinib (14). However, this could have been a reflection of small sample size rather than lack of effect. There is limited data for the utility of imatinib in the palliative setting. Within a registry of 823 Korean patients with GISTs, 35 patients were found to have mutations in PDGFRα. Of these, only 4 had non D842V exon 18 mutations. All 4 patients achieved a partial response to first line imatinib and only one patient of these 4 went on to second line therapy with sunitinib. Of note, that patient did at least achieve stable disease (33).
Exon 12
Mutations in exon 12 are rare, but do represent the third most common mutation in PDGFRα. Patients with this mutation seem to have similar benefits to first line imatinib as the non-D842V exon 18 mutations. In a group of 8 patients with exon 12 mutations, 7 of 8 proved to have clinical benefit to imatinib (1 complete response, 3 partial responses, and 3 with stable disease). The benefit of these responses mirrors that seen in KIT exon 11 mutations with a median PFS of approximately 2 years (34). Benefit from any second line palliative treatments, including sunitinib, seems to be limited with progression free survival averaging 2 months (34). There are, however, cases of patients with exon 12 mutations who have achieved prolonged responses to sunitinib (35). There is insufficient data and experience with regorafenib in this population to help guide clinical decision making.
Wild type GIST
GISTs without driver mutations in KIT or PDGFRA traditionally have been grouped together as wild-type GISTs. However, among the KIT/PDGFRA wild-type GISTs, there exist molecularly distinct sub-groups (36). Between 20–40% have been found to be deficient for the succinate dehydrogenase (SDH) ubiquinone complex (SDHA, SDHB, SDHC, SDHD) (36). In addition, a fusion of ETV6-NTRK3 has been identified in a subset of wild-type GIST which may have therapeutic implications (37). Finally, another 15% carry mutations in the RAS-RAF signaling pathway, most commonly BRAF. The remainder then fall into the KIT/PDGFRα/RAS/SDH wild-type or ‘quadruple wild-type’ GISTs (38). Standard of care for wild-type GIST should be sarcoma center referral and clinical trial.
As a group, wild-type GISTs have mixed response rates to first line TKIs, likely reflecting the heterogeneous nature of this category. Reported response rates to palliative imatinib are low (12,22). Further, in the adjuvant setting, it does not appear that imatinib offers any benefit for wild-type GIST and is not routinely offered (21). Sunitinib, however, has shown activity with clinical benefit in 56% of wild-type patients and progression free survival averages of 19 months, mirroring the benefit seen in KIT exon 9 patients (23). In the phase II regorafenib study, 8 wild-type GIST patients were included and had similar PFS to KIT exon 9 or 11 mutations (39).
Performing comprehensive genomic analysis of these tumors may be reasonable to search for molecular targets or opportunities for clinical trial enrollment. For example, there has been at least one report of a clinical response to dabrafenib in a V600E BRAF mutant GIST (40).
Future therapies
As research into the understanding of GIST continues, three areas of current development are of interest: (I) pursuing new targets such as the transcription factor ETV1 through novel MEK inhibitors; (II) immunotherapy combinations with TKIs; and (III) the development of metabolic based therapies.
ETV1 is highly expressed in GISTs, regardless of mutational status. ETV1 seems to have a synergistic relationship with downstream KIT signaling through the MAP kinase pathway. Additionally, ETV1 has been demonstrated to be necessary for GIST cell survival (41). In mouse models, the combination of imatinib and a MEK inhibitor, MEK162, demonstrated near complete clinical responses. Phase Ib studies in imatinib refractory patients have been completed, and a phase II study in treatment naïve patients is ongoing (42).
A role for immune based therapy is in the early stages of investigation. The combination of ipilumumab with dasatinib was tested in 8 GIST patients who had progressed after at least 1 line of therapy. In this study, one patient had a durable response lasting over a year (59+ weeks). Plans for a phase II investigation are underway (42). The combination of ipilumumab and imatinib is also in the early phases of investigation (NCT01738139).
Finally, metabolism is also an area of active investigation for the treatment of wild-type and cKIT/PDGFRα GIST. Given that succinate dehydrogenase deficiency is seen in many wild-type GISTs, and is thought to increase glutamine addiction, glutaminase inhibition using CB-839 is currently being studied in clinical trial NCT02071862. Finally, loss of expression of argininosuccinate synthetase 1 (ASS1) has been found in 97% of GIST (43). This metabolic deficiency, which is also common in other sarcomas, may allow for a new avenue of therapeutic opportunities as multi-agent therapies based on this deficiency using PEGylated arginine deiminase (ADI-PEG20) are currently being pursued.
Conclusions
The management of GISTs has evolved rapidly over the past two decades, driven by an ever-growing understanding of the disease’s molecular diversity. Today in the clinic, discussion of treatment options in both the adjuvant and metastatic settings is influenced by the information obtained by sequencing of the KIT and PDGFRα genes. As we look to the future, clinical trial development will become more molecularly focused, specifically looking for targets in each subset of the GIST population.
Acknowledgements
None.
Footnote
Conflicts of Interest: BVT has been an advisor to Novatris.
References
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Edmonson JH, Marks RS, Buckner JC, et al. Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest 2002;20:605-12. [Crossref] [PubMed]
- Oncology NCPGi. Soft Tissue Sarcoma. National Comprehensive Cancer Network; [May 28, 2016]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Uçar AD, Oymaci E, Carti EB, et al. Characteristics of Emergency Gastrointestinal Stromal Tumor (GIST). Hepatogastroenterology 2015;62:635-40. [PubMed]
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19. [Crossref] [PubMed]
- Isozaki K, Hirota S, Nakama A, et al. Disturbed intestinal movement, bile reflux to the stomach, and deficiency of c-kit-expressing cells in Ws/Ws mutant rats. Gastroenterology 1995;109:456-64. [Crossref] [PubMed]
- West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107-13. [Crossref] [PubMed]
- Barnett CM, Corless CL, Heinrich MC. Gastrointestinal stromal tumors: molecular markers and genetic subtypes. Hematol Oncol Clin North Am 2013;27:871-88. [Crossref] [PubMed]
- Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol 2008;26:5360-7. [Crossref] [PubMed]
- Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006;42:1093-103. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016;34:244-50. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [Crossref] [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [Crossref] [PubMed]
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563-70. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352-9. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 2006;24:4764-74. [Crossref] [PubMed]
- Prenen H, Cools J, Mentens N, et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin Cancer Res 2006;12:2622-7. [Crossref] [PubMed]
- Patrikidou A, Domont J, Chabaud S, et al. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer 2016;52:173-80. [Crossref] [PubMed]
- Lasota J, Corless CL, Heinrich MC, et al. Clinicopathologic profile of gastrointestinal stromal tumors (GISTs) with primary KIT exon 13 or exon 17 mutations: a multicenter study on 54 cases. Mod Pathol 2008;21:476-84. [Crossref] [PubMed]
- Garner AP, Gozgit JM, Anjum R, et al. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clin Cancer Res 2014;20:5745-55. [Crossref] [PubMed]
- Evans EK, Hodous BL, Gardino AK, et al. Abstract 791: BLU-285, the first selective inhibitor of PDGFRα D842V and KIT Exon 17 mutants. Cancer Research 2015;75:Abstr 791.
- Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005;23:5357-64. [Crossref] [PubMed]
- Sadovnik I, Lierman E, Peter B, et al. Identification of Ponatinib as a potent inhibitor of growth, migration, and activation of neoplastic eosinophils carrying FIP1L1-PDGFRA. Exp Hematol 2014;42:282-293.e4. [Crossref] [PubMed]
- Heinrich MC, Griffith D, McKinley A, et al. Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin Cancer Res 2012;18:4375-84. [Crossref] [PubMed]
- Yoo C, Ryu MH, Jo J, et al. Efficacy of Imatinib in Patients with Platelet-Derived Growth Factor Receptor Alpha-Mutated Gastrointestinal Stromal Tumors. Cancer Res Treat 2016;48:546-52. [Crossref] [PubMed]
- Cassier PA, Fumagalli E, Rutkowski P, et al. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res 2012;18:4458-64. [Crossref] [PubMed]
- Brohl AS, Demicco EG, Mourtzikos K, et al. Response to sunitinib of a gastrointestinal stromal tumor with a rare exon 12 PDGFRA mutation. Clin Sarcoma Res 2015;5:21. [Crossref] [PubMed]
- Boikos SA, Pappo AS, Killian JK, et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2016;2:922-8. [Crossref] [PubMed]
- Brenca M, Rossi S, Polano M, et al. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol 2016;238:543-9. [Crossref] [PubMed]
- Pantaleo MA, Nannini M, Corless CL, et al. Quadruple wild-type (WT) GIST: defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med 2015;4:101-3. [Crossref] [PubMed]
- George S, Wang Q, Heinrich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol 2012;30:2401-7. [Crossref] [PubMed]
- Falchook GS, Trent JC, Heinrich MC, et al. BRAF mutant gastrointestinal stromal tumor: first report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget 2013;4:310-5. [Crossref] [PubMed]
- Ran L, Sirota I, Cao Z, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov 2015;5:304-15. [Crossref] [PubMed]
- Shoushtari AN, D'Angelo SP, Keohan ML, et al. Combined KIT and CTLA-4 blockade in patients with refractory GIST and other advanced sarcomas. J Clin Oncol 2014;32:Abstr 10521.
- Bean GR, Kremer JC, Prudner BC, et al. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death Dis 2016;7:e2406. [Crossref] [PubMed]


