Recent advances and significance of intra-arterial infusion chemotherapy in non-resectable colorectal liver metastasis
Introduction
Worldwide, liver metastases develop in 50% of patients with colorectal carcinoma and colorectal liver metastases (CLM) are currently thought to represent a major health problem (1). At this stage, conventional criteria for resectability include presence of less than four metastases, unilobar distribution, maximum tumor size less than 5 cm, good functional reserve of the liver and potential for complete resection (2-4). As a result, approximately 70-80% of patients with CLM are assigned a non-resectable status (5,6). For patients who do not undergo hepatectomy, survival rates have been poor, with 5-year survival rates less than 40%, although use of novel chemotherapeutic regimens such as oxaliplatin, irinotecan (CPT-11) and molecular-targeting drugs (e.g., cetuximab or bevacizumab) has increased the median survival for such patients (7-10). The potential value of resectability in achieving long-term survival has resulted in the development of oncological strategies for initially non-resectable CLM. Adam et al. reported a 13% conversion rate to resectability of non-resectable CLM after downsizing by effective chemotherapy in select cases, associated with a 5 year-survival rate of 33% after conversion hepatectomy (11). Even in those few patients who underwent hepatectomy, tumor relapse in the remnant liver appears frequent and indications for repeat hepatectomy are limited (12-14). Most patients with recurrent CLM also need chemotherapy similar to those with non-resectable CLM.
With a traditional regimen of 5-fluorouracil (5-FU) and leucovorin (LV), tumor response rate is approximately 20% and median survival with non-resectable CLM is 12 months (15,16). Modern regimens such as combined 5-FU/LV with oxaliplatin or CPT-11 have achieved response rates of approximately 50% (17), and median survival of non-resectable CLM patients has increased to 20-23 months (18,19). Furthermore, with the development of biological agents such as cetuximab or bevacizumab, tumor response rates and median survival have continued to increase (9,10,20,21). Given these effective chemotherapeutic regimens, major tumor shrinkage can be achieved in some CLM patients, but complete response (CR) is rare. In addition, the new systemic chemotherapeutic regimens have been associated with skin reactions, high costs and impaired liver functions (22,23). Furthermore, in CLM patients with extrahepatic metastasis, control of liver metastases might be related to overall survival (24). To solve this problem and improve control of non-resectable CLM, we have been attempting hepatic intraarterial infusion chemotherapy (HAIC) since 2000, as have other groups (25,26). Local control using HAIC has appeared remarkable. In cases where control of liver metastases is a major goal for improving prognosis, the role of HAIC remains unclear.
The present study examined treatment results for HAIC in 36 patients with non-resectable CLM and tumor relapse in the liver after hepatectomy to clarify treatment efficacy, clinical benefit and limitations.
Patients and methods
Patients and follow-up
Thirty-six consecutive patients (25 males, 11 females) with non-resectable CLM with or without extrahepatic metastases who were admitted to the Division of Surgical Oncology, Department of Surgery, Nagasaki University Graduate School of Biomedical Sciences (NUGSBS) between 2000 and 2009 were analyzed retrospectively in this study. Synchronous CLM with primary colorectal tumor was observed in 16 patients, metachronous CLM in 5 and posthepatectomy recurrence of CLM in 15. Chemotherapeutic regimens for HAIC comprised 5-FU continuous intraarterial infusion (CIA) in 11 patients, irinotecan (CPT-11) in 16 and the combination of both in 9. Detection and follow-up imaging were performed using multi-detector computed tomography (CT) or magnetic resonance imaging (MRI) every 3-6 months and serum levels of carcinoembryonic antigen (CEA) measured every month during follow-up. The entire study design was approved by the Human Ethics Review Board of our institution. Informed consent for data collection was obtained from each patient prior to enrolment. Patient data were retrieved from the NUGSBS database.
Definition of non-resectable CLM and treatment protocol for chemotherapy
Our Nagasaki criteria of non-resectable CLM comprise: (I) numerous liver metastases, but the number is not clearly defined; (II) small functional liver volume (remnant volume <30% or <300 cm3) was estimated when major hepatectomy was considered; (III) poor functional liver reserve evaluated by indocyanine green retention rate at 15 min or 99m-technetium-galactosyl serum albumin liver scintigraphy (27); and (IV) massively progressed extrahepatic metastases. Clinical parameters were defined according to the Japanese Classification of Colorectal Carcinoma (28).
In cases of resectable CLM, 6-8 cycles of the modified FOLFOX6 with or without cetuximab or bevacitumab was used as a neoadjuvant setting for multiple CLM over 4 regions. Adjuvant chemotherapy after hepatectomy comprised oral administration of UFT (tegafur-uracil; Taiho Pharmaceutical Co., Tokyo, Japan) plus l-leocovorin (Takeda Chemical Industries, Tokyo, Japan), or S-1 (Taiho Pharmaceutical Co.) or capecitabine (Xeloda; Roche, Nutley, NJ). In case of H2- or H3-grade CLM according to Japanese criteria (tumor size >5 cm, or number of tumors >4), 4-6 cycles of the modified FOLFOX6 with or without cetuximab or bevacizumab was administered after hepatectomy. In cases where recurrent tumor was able to be resected, repeat radical hepatectomy was selected.
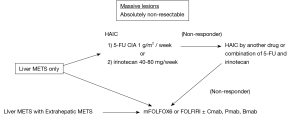
Chemotherapeutic regimens for non-resectable CLM and recurrent non-resectable CLM are shown in Figure 1. For CLM showing massive liver metastases without extrahepatic metastases, HAIC was selected. The first-line regimen is 1 g/m2 of 5-FU CIA and the second-line regimen is 5-FU CIA plus 40-80 mg of CPT-11 per week. In cases where first- and second-line HAIC regimens elicited no response, systemic chemotherapy comprising modified FOLFOX 6 or FOLFIRI with or without molecular targeting drugs was applied concurrent with HAIC. In cases of non-resectable CLM with extrahepatic metastases, HAIC was generally not selected.
Statistical analysis
Tumor-free and overall survival and time to progression after treatment were calculated according to the Kaplan-Meier method, and differences between groups were tested for significance using the log-rank test. A two-tailed P value <0.05 was considered as significant. All statistical analyses were performed using SPSS version 18.0 software (SPSS, Chicago, IL).
Results
Survival after HAIC for non-resectable CLM
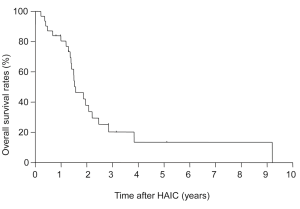
Progression-free survival after IAIC was 10.8 months. Figure 2 shows survival after IAIC in cases with non-resectable CLM. The 1-, 3- and 5-year survival rates after HAIC were 84%, 21% and 13%, respectively, and median survival after IAIC was 32.5 months. Tumor response after HAIC was CR in 4 patients (11%), partial response (PR) in 19 (53%), stable disease (SD) in 6 (17%) and progressive disease (PD) in 7 (19%). Disease control rate was 81% and response rate was 64%. Two cases showing PR became resectable from non-resectable CLM after decreasing the number of tumors although conversion hepatectomy was eventually not performed.
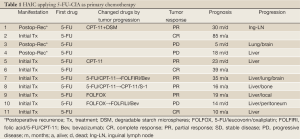
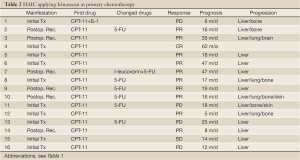
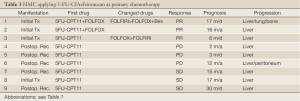
Table 1 shows treatment results of HAIC using 5-FU CIA as a primary chemotherapy in 11 patients. Eight patients underwent HAIC for non-resectable CLM and three underwent HAIC for posthepatectomy recurrence. Six cases received second-line chemotherapy, including HAIC with CPT-11 in two, HAIC as a combination of 5-FU and CPT-11 in two, and systemic chemotherapy in two. Three cases received third-line chemotherapy, including HAIC plus S-1 oral administration in one, and systemic chemotherapy with bevacizumab in two. CR was observed in 3 of 11 patients (27%), PR in 5 (46%), and PD in 3. Two of 3 cases showing CR achieved long survival without tumor relapse. All cases showing PR and PD had tumor progression, but two cases survived over 24 months. Table 2 shows the treatment results of HAIC using CPT-11 (irinotecan) as a primary chemotherapy in 16 patients. Ten patients underwent HAIC for non-resectable CLM and 6 underwent HAIC for posthepatectomy recurrence. Seven cases received second-line chemotherapy, including HAIC with 5-FU CIA in 6, and systemic chemotherapy in 1. CR was observed in 1 of 16 patients (6%) (Figure 3), PR in 10 (63%), SD in 1 and PD in 4. One patient showing CR achieved long survival without tumor relapse. All patients except the one showing CR displayed tumor progression, but 3 cases showing PR achieved survival over 24 months. Table 3 shows treatment results for HAIC using a combination of 5-FU CIA and CPT-11 (irinotecan) as a primary chemotherapy in 9 patients. Four patients underwent HAIC for non-resectable CLM and 5 underwent HAIC for posthepatectomy recurrence. Two of 9 cases (22%) received second-line systemic chemotherapy. CR was not observed and PR was observed in 3 patients (33%), SD in 3 and PD in 3. All patients showed tumor progression and only 1 patient showing SD survived over 24 months.
Full table
Full table
Full table
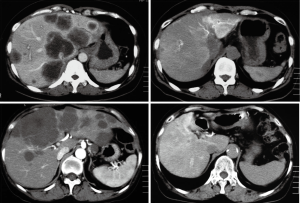
Table 4 shows morbidity after HAIC, with 6 patients displaying associated complications (17%). Median duration of HAIC use was 238 days. Cather occlusion was observed in 3 patients, port-site infection in 2 and catheter dislocation in 1. HAIC was able to be continued in 4 of these 6 cases by re-inserting or exchanging the catheter. Chemotherapy-associated complications were blood toxicity with grade 1 or 2 in 13 patients. Grade 4 leukocytopenia was observed in 2 patients (6%), one of whom died from subsequent acute respiratory distress syndrome and sepsis.

Full table
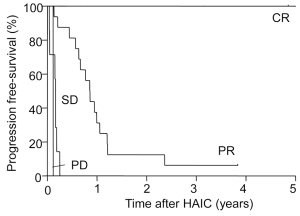
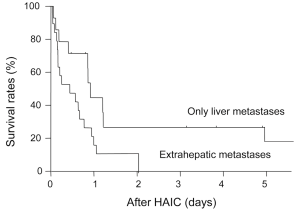
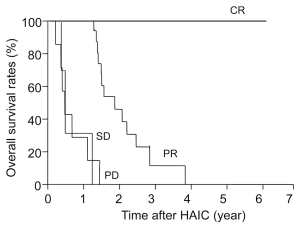
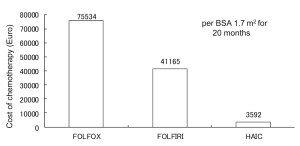
Figure 4 shows tumor progression-free survival after HAIC for each level of response to chemotherapy. Median survival in CR patients was 57 months and no tumor progression was seen; survival was significantly longer than that with PR (13 months, P=0.024), SD (1.7 months, P=0.012) or PD (1.5 months, P=0.016). Figure 5 shows tumor progression-free survival in patients with or without extrahepatic metastases. Patients with only intrahepatic CLM achieved significantly longer survival (15.8 months) than those with extrahepatic metastases (9.8 months, P=0.047). Figure 6 shows overall survival after HAIC with each level of response to chemotherapy. Median survival period with CR was 62.3 months and all survived; survival was significantly longer than that with PR (25.4 months, P=0.021), SD (12.1 months, P=0.018) or PD (8.4 months, P=0.014). No patients showing PR, SD or PD survived over 50 months. Figure 7 shows a comparison of medical fees per 1.7 m2 of body surface area for 20 months of HAIC and systemic chemotherapy at our institute (in Euros). Cost of FOLFOX was 21-time higher and FOLFIRI was 11-times higher than that of HAIC (P<0.01).
Discussion
In the era of systemic chemotherapy for CLM, the clinical significance of HAIC was not noted worldwide because of the similar survival benefit, reduced effectiveness against extrahepatic metastases and complicated management or catheter-associated problems (29). Kerr et al. reported that no survival benefit of HAIC has been found with the development of improved regimens of systemic chemotherapy (30). They concluded that no evidence for any survival advantage with HAIC was observed and continued use of this regimen was not recommended outside of clinical trials. Other reports have likewise denied the clinical utility of HAIC in comparison with intravenous systemic chemotherapy (31,32). However, the regimen of drugs for HAIC was limited in these reports and no evaluations of continuous infusion of 5-FU or irinotecan had been undertaken. In the report by Kerr, dropout from the HAIC group due to catheter-related problems was relatively many, at 39%, and 51% of subjects did not achieve administration of 6 cycles. Despite this lack of ability to manage HAIC, median overall survival was comparable between HAIC and systemic chemotherapy (HAIC, 14.7 months; systemic chemotherapy, 14.8 months; hazard ratio, 1.04). A comparison of complications and survival benefits under adequate management of chemotherapy is therefore warranted. HAIC has still been applied in some institutes, including our own. Benefits for high response rate including complete diminishing of tumor image, longer survival, and lower cost in comparison with systemic chemotherapy were identified in the present study, suggesting that this treatment modality may be useful for controlling CLM.
In the present study, CR was observed in 4 patients (11%) and total response rate was high, representing a satisfactory result. In particular, patients with CR showed a long period of CR and long overall survival. In patients receiving systemic chemotherapy, the rate of achieving CR is supposed to be low at this stage (33). The power of local control with HAIC thus appears promising. Kemeny et al. reported on the CALGB9481 test, as a randomized prospective trial between groups receiving HAIC with FUDR and leucovorin compared to systemic chemotherapy with 5-FU and leucovorin (34). Their results showed a significantly longer median survival (24.4 months), longer progression-free survival (9.8 months), and higher response rate (47%) with HAIC in comparison with systemic chemotherapy. The present results were similar to those described by Kemeny et al., albeit with a higher response rate of 64% (34). This might be attributable to different regimens of chemotherapy. In comparison with the latest systemic chemotherapy, survival and response rate in our results were not unfavourable (18,22,33). Although catheter-related problems were emphasized in previous results (29,30) and we also encountered 6 cases with catheter-related complication, HAIC was able to be maintained in 4 cases with replacement of a port or catheter. In comparison with the report by Kerr et al. (30), the complication rate was low and management was better in our study. When the management of ports and catheters for HAIC was well-organized, the scheduled cycle of administration of HAIC would be achievable in many cases. In terms of severe chemotherapy-related toxicity, we encountered only 2 patients. The drug toxicity of HAIC is lower than that of FOLFOX, FOLFIRI or use of molecular-targeted drugs (35).
In non-CR cases, tumors eventually progressed and patients died within 4 years. Furthermore, CLM with extrahepatic metastases showed very poor prognosis. Additional methods to obtain longer survival are thus necessary in such cases. We attempted combination therapy with HAIC and systemic chemotherapy to improve survival in non-CR cases. As HAIC was relatively inexpensive and showed fewer severe side effects compared to FOLFOX or FOLFIRI in our results, the significance of HAIC for controlling liver metastases remains. By combining systemic chemotherapy with HAIC, a well-balanced regime for better quality results may be achieved. Kemeny et al. reported the significance of HAIC with systemic chemotherapy for non-resectable CLM, in combination with oxaliplatin/CPT-11/FUDR. The response rate reached high as 90%, and median survival was long, at 36 months as bove (36). Ducreux et al. also reported the combination of HAIC and systemic chemotherapy with oxaliplatin/5-FU/leucovorin, in which response rate was 64% and median survival was 27 months. Efficacy of the trial by Kemeny was superior to that of systemic chemotherapy or that of HAIC alone (36). Although control of extrahepatic metastasis by HAIC was weak, cause of death may be due to intrahepatic tumor progression. HAIC thus remains a useful chemotherapeutic option at this stage (37).
In conclusion, HAIC showed a high response rate and 4 cases of CR with long survival despite non-resectable CLM. Although catheter-related complications were observed in 17%, HAIC was able to be continued in 4 of the 6 cases and no severe drug toxicity was observed. From the perspective of view medical cost, HAIC appears cost-effective in comparison with recent systemic chemotherapies. HAIC for non-resectable CLM together with recent advances in systemic chemotherapy appears useful. To achieve good control of non-resectable CLM in the absence of extrahepatic metastases, HAIC can have a major impact with high anti-cancer response and prolonged survival, which can be applied to conversion hepatectomy in some groups with better responses to HAIC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg 1989;210:127-38.
- Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Registry of Hepatic Metastases. Surgery 1988;103:278-88.
- Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59-71.
- Figueras J, Torras J, Valls C, et al. Surgical resection of colorectal liver metastases in patients with expanded indications: a single-center experience with 501 patients. Dis Colon Rectum 2007;50:478-88.
- Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol 2003;14:ii13-6.
- Wicherts DA. Chapter 1. general outline and introduction of the thesis. In: Wicherts DA. eds. New strategies for advanced colorectal liver metastases. No more a fatality. Enschede, Netherlands: Gildeprint Drukkerijen. 2011:10-7.
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66.
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7.
- Folprecht G, Lutz MP, Schöffski P, et al. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol 2006;17:450-6.
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45.
- Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 2004;240:1052-61; discussion 1061-4.
- Lopez P, Marzano E, Piardi T, et al. Repeat hepatectomy for liver metastases from colorectal primary cancer: a review of the literature. J Visc Surg 2012;149:e97-e103.
- Sa Cunha A, Laurent C, Rault A, et al. A second liver resection due to recurrent colorectal liver metastases. Arch Surg 2007;142:1144-9; discussion 1150.
- Yamamoto J, Kosuge T, Shimada K, et al. Repeat liver resection for recurrent colorectal liver metastases. Am J Surg 1999;178:275-81.
- Thirion P, Michiels S, Pignon JP, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol 2004;22:3766-75.
- Pohlen U, Mansmann U, Berger G, et al. Multicenter pilot study of 5-fluorouracil, folinic acid, interferon alpha-2b and degradable starch microspheres via hepatic arterial infusion in patients with nonresectable colorectal liver metastases. Anticancer Res 2004;24:3275-82.
- Boyle P, Leon ME. Epidemiology of colorectal cancer. Br Med Bull 2002;64:1-25.
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670-6.
- Köhne CH, van Cutsem E, Wils J, et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol 2005;23:4856-65.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42.
- Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38-47.
- Gotlib V, Khaled S, Lapko I, et al. Skin rash secondary to bevacizumab in a patient with advanced colorectal cancer and relation to response. Anticancer Drugs 2006;17:1227-9.
- Tol J, Koopman M, Rodenburg CJ, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol 2008;19:734-8.
- Stillwell AP, Ho YH, Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J Surg 2011;35:684-92.
- Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol 2008;15:219-26.
- Ducreux M, Ychou M, Laplanche A, et al. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: a trial of the gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol 2005;23:4881-7.
- Nanashima A, Tobinaga S, Abo T, et al. Reducing the incidence of post-hepatectomy hepatic complications by preoperatively applying parameters predictive of liver function. J Hepatobiliary Pancreat Sci 2010;17:871-8.
- Japanese Society for Cancer of the Colon and Rectum. 3.2.2 In: Mutoh T. eds. General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus (in Japanese). 7th ed. Tokyo: Kanehara & Co., Ltd., 2006:14-5.
- Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg 2005;201:57-65.
- Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet 2003;361:368-73.
- Mocellin S, Pilati P, Lise M, et al. Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J Clin Oncol 2007;25:5649-54.
- Chan R, Kerr D. Hepatic arterial chemotherapy for colorectal cancer liver metastases: a review of advances in 2003. Curr Opin Oncol 2004;16:378-84.
- Aparicio J, Fernandez-Martos C, Vincent JM, et al. FOLFOX alternated with FOLFIRI as first-line chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer 2005;5:263-7.
- Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24:1395-403.
- Seki H, Ozaki T, Shiina M. Hepatic arterial infusion chemotherapy using fluorouracil followed by systemic therapy using oxaliplatin plus fluorouracil and leucovorin for patients with unresectable liver metastases from colorectal cancer. Cardiovasc Intervent Radiol 2009;32:679-86.
- Kemeny N, Jarnagin W, Paty P, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol 2005;23:4888-96.
- Kanat O, Gewirtz A, Kemeny N. What is the potential role of hepatic arterial infusion chemo-therapy in the current armamentorium against colorectal cancer. J Gastrointest Oncol 2012;3:130-8.