Is surgery mandatory in locally advanced gastrointestinal stromal tumors after imatinib? A case report and literature review
Introduction
Gastrointestinal stromal tumors (GISTs) are relatively rare mesenchymal neoplasms of the gastrointestinal tract; due to the rarity of esophageal GISTs (about 2% of all GISTs), their treatment and clinical outcome are not completely clear. A radical surgery with negative microscopic margins is the standard treatment of all GISTs (1). Nevertheless, in some cases, the lesion cannot be surgically removed, due to the mass volume or to specific anatomical contacts or locations. About 80% of newly diagnosed cases are resectable at presentation; in patients with unresectable disease at diagnosis imatinib mesylate (IM), a small molecule inhibitor of the GIST oncoprotein KIT and PDGFR-alpha, has a proven efficacy (2). Recent studies have suggested that pre-operative IM could lead to a R0 tumor resection in 83% of cases with a median overall survival (OS) of 104 months (3). We report a case of an unresectable esophageal GIST harboring a KIT exon 11 mutation [frequency of KIT exon 11 mutations in KIT-mutated GIST is about 70% (4)] treated with neoadjuvant IM who obtained an excellent response and a subsequent radical surgical resection.
Case presentation
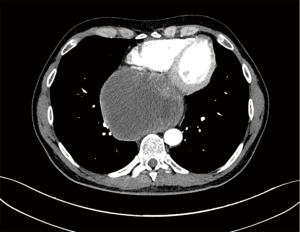
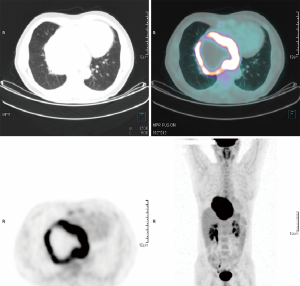
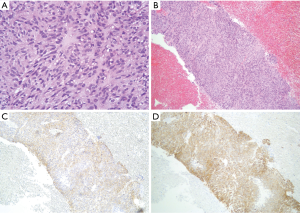
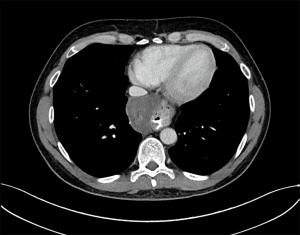
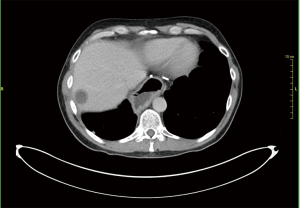
A 46-year-old Caucasian male referred to our hospital in June 2014 for pain in lower chest and epigastric area, anorexia and subsequent weight loss of 8 kilograms. He had no comorbidities except for a tubercular pleurisy 20 years ago. Oesophagogastroduodenoscopy (OGD) and computed tomography (CT) demonstrated a partially obstructing mass of 10×9×12 cm3 in the esophagus-gastric junction with extension in posterior mediastinum and compression of the left atrium and of the right inferior pulmonary vein (Figure 1); no metastases were evident. Positron emission tomography (PET)-CT confirmed the giant mass with an increased metabolic activity in the posterior mediastinum [standardized uptake value (SUV) 21.2] with a central photopenic area as for necrosis, in absence of further captation areas (Figure 2). Pulse rate was 70/min, blood pressure 120/80 mmHg and respiratory rate 15/min; the physical examination showed only collateral circulation veins in the upper chest wall. Biopsy revealed a spindle-cell GIST with diffuse positivity of CD34 and CD117 and a deletion in exon 11 of c-Kit (ΔW557_V559> F) (Figure 3). The tumor was considered unresectable, so, since GISTs carrying mutation in exon 11 are sensible to IM, in August 2014 the patient started a neoadjuvant treatment with IM 400 mg/die. Medical examinations were performed monthly to evaluate treatment tolerability and CT scan was repeated every 2 months in order to identify the maximum response. After 9 months of therapy, restaging CT scan performed in April 2015 showed a reduction of the tumor mass of 83%, measuring 63×38×75 mm3 (Figure 4). CT scan performed in August 2015 showed no further volume reduction, so patient was newly evaluated for surgery in multidisciplinary team and in September 2015 underwent Ivor Lewis oesophagogastrectomy. The mass did not infiltrate surrounding structures and no tumor rupture was experienced during surgery. Pathologic examination of surgical specimen revealed a mass of 7 cm with hyaline-regressive areas and white nodules of residual tumor measuring 2.2 cm; at microscopic view, lymph nodes and margins were negative. A new evaluation of tumor sample confirmed the mutation in exon 11 without any change in molecular pattern. In October 2015 patient restarted IM 400 mg/die as adjuvant treatment but the CT scan performed in May 2016 showed the appearance of a liver metastasis (Figure 5). IM was stopped and the liver metastasis was treated with radiofrequency ablation.
Discussion
The use of IM in neoadjuvant setting is relatively recent, the studies available are limited and the sample size of patients enrolled is small. Preoperative IM is useful to reduce the tumor volume, to improve the surgical outcome given the high morbidity and mortality of surgical resections, but no definitive data are available about improvement of OS. Since esophageal origin of GISTs is associated with poor prognosis mainly due to the primary site, the role of neoadjuvant IM could be particularly interesting in order to allow radical surgery improving survival outcomes.
Few studies support the use of IM in neoadjuvant setting and many of them do not include esophageal GISTs because of their rarity. A phase II prospective trial from Eisenberg et al. evaluated 2–3 months of neoadjuvant IM treatment in 52 patients, 30 with locally advanced GISTs and 22 with metastatic disease (2); no one had an esophageal primary location. The pre-operative response in locally advanced GISTs by RECIST (5) was partial in 7% of patients, stable in 83%, and unknown in 10%; in most cases (77%) a R0 resection was obtained, 15% of patients had R1 resection and 8% R2 resection. The 2-year estimated progression-free survival (PFS) was 82.7% and the 2-year estimated OS 93.3%. Long-term follow-up results showed a 5-year PFS of 56% and a 5-year OS of 77%; no correlation between surgical resection status and tumor progression was found either in recurrent/metastatic and in locally advanced disease (no surgery, R1–2: 60% vs. R0: 23.8%; P=0.11) (6). Another phase II prospective study, the APOLLON trial (7), evaluated the overall tumor response and progression rate of disease in 41 locally advanced, non-metastatic GISTs (10 out of 41 with oesophageal disease); R0 resections were performed in 88.2% of patients and the PFS rate at 3 years was 85.2%. Mean time to progression (TTP) was 64.2 months (95% CI, 55.6–72.6), mOS was 74.9 months (95% CI, 69.1–80.6). The role of neoadjuvant IM was also evaluated in a subgroup of patients affected by locally advanced GISTs (25/434) enrolled in the phase III BFR14 trial; only two patients had oesophageal GIST (8). Median time of neoadjuvant IM treatment was 7.3 months; 60% of patients obtained a partial response, but only 36% underwent surgical resection. Median PFS was 32.1 months, while median OS was not reached after 53.5 months of follow-up. Nevertheless, analyzing only the 15 patients who had partial response to IM, no statistically significant difference in PFS was observed between resected and unresected patients (P=0.2829). Shen et al. evaluated a small consecutive series of 18 patients with locally advanced or recurrent/metastatic unresectable GISTs treated with preoperative IM for a median time of 7 months; no oesophageal GISTs were included (9). Fifty percent of patients underwent surgery; after preoperative therapy 88.9% showed a partial response and 11.1% a stable disease (according to criteria different from RECIST). No data about survival analysis were provided. The study from Fiore et al. (10) showed a high rate of tumor shrinkage in a series of 15 patients (only one with oesophageal disease) treated with pre-operative 9-month IM with a median size reduction of 34%; PFS at 3 years was 77%. In a large retrospective analysis from Rutkowski et al. (3), 161 patients with locally advanced unresectable GISTs treated with neoadjuvant IM for a median time of 40 weeks were evaluated; 3% of patients had oesophageal tumor. In 83% of cases it was obtained a R0 resection and only two patients experienced progressive disease during IM treatment; 5-year disease specific survival (DSS)/disease-free survival (DFS) rates were 95%/65%, respectively, median OS was 104 months.
Unfortunately, no definitive conclusions can be drawn due to the significant limitations and heterogeneity of the studies above reported: small sample size, different primary sites, patients’ selection (potentially resectable at diagnosis vs. unresectable locally advanced vs. recurrent/metastatic GISTs), duration of preoperative therapy which is often prolonged on the basis of tumor response from 3 to 12 months (11,12).
Current recommendations for assessing the risk of progression for a newly diagnosed primary GIST rely on tumor size, tumor location and mitotic index [mitoses per 50 high-power fields (HPF)] (13,14). In our case the risk of recurrence was high since it was an oesophageal GIST larger than 10 cm (independently from HPF). Moreover, the tumor was in anatomical contact with left atrium and the right inferior pulmonary vein; this implied a high operative risk either for morbidity and mortality. Our patient was treated with neoadjuvant IM for 12 months with a reduction of the tumor mass of 83%. Nevertheless, the maximum response to treatment was obtained in the first 9 months of therapy (from August 2014 to April 2015) without any further reduction of the mass in the subsequent 3 months; in fact, the last CT scan performed in August 2015 showed a stable disease if compared with April 2015, as for acquired resistance to therapy. However, no secondary mutations were found out at a second analysis of surgical specimens. It is noteworthy that our patient relapsed after 8 months from oesophagogastrectomy, during IM adjuvant treatment.
To date, prospective studies did not provide definitive results about significance of preoperative IM and surgical resection in survival outcomes of patients affected by locally advanced GISTs. Thus in high risk resections a balance between morbidity and mortality derived from surgery should be carefully considered before embarking on a major surgical procedure. So larger studies with strict inclusion criteria (tumor location, initial tumor size, KIT/PDGFRA mutational status, mitotic index, standardized duration of pre-operative IM, correct timing of surgery before the onset of acquired resistance) are needed to identify patients affected by locally advanced GISTs that could most benefit from surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Lee HJ, Park SI, Kim DK, et al. Surgical resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2009;87:1569-71. [Crossref] [PubMed]
- Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009;99:42-7. [Crossref] [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012;19:1074-80. [Crossref] [PubMed]
- Hohenberger P, Langer C, Wendtner CM, et al. Neoadjuvant treatment of locally advanced GIST: Results of APOLLON, a prospective, open label phase II study in KIT- or PDGFRA-positive tumors. J Clin Oncol 2012;30:abstr 10031.
- Blesius A, Cassier PA, Bertucci F, et al. Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer 2011;11:72. [Crossref] [PubMed]
- Shen C, Chen H, Yin Y, et al. Preoperative imatinib for patients with primary unresectable or metastatic/recurrent gastrointestinal stromal tumor. Clinics (Sao Paulo) 2014;69:758-62. [Crossref] [PubMed]
- Fiore M, Palassini E, Fumagalli E, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol 2009;35:739-45. [Crossref] [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [Crossref] [PubMed]
- Tielen R, Verhoef C, van Coevorden F, et al. Surgery after treatment with imatinib and/or sunitinib in patients with metastasized gastrointestinal stromal tumors: is it worthwhile? World J Surg Oncol 2012;10:111. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 2008;3:557-86. [Crossref] [PubMed]


