Investigation of the human H3.3B (H3F3B) gene expression as a novel marker in patients with colorectal cancer
Introduction
Histones are a family of basic proteins that associate with DNA and help it to condense into chromatin. This family of proteins has five classes that include: H1, H2A, H2B, H3, and H4. All classes consist of several subtypes except H4 (1,2). Histone genes are classified into three groups. The first group includes the replication-dependent histones that are express only at the S phase of the cell cycle. The second group includes replication-independent histones that are express in all phases of the cell cycle, and the last one is testis-specific histones that express only in the testicular tissue (2). Histone H3 family include a large family of histones that contain H3.1, H3.2, H3.3 (H3F3), CENP-A/cenH3 and testis-specific histones like H3t (3). H3.3 histone is a replacement histone subtype that is express in entire cell cycle phases (it is independently express from S phase) and overexpress in transcriptionally active regions, promoter regions, intergenic or intragenic regulatory elements, and heterochromatin regions of telomeric, subtelomeric and pericentromeric regions (helping to protect and maintenance the integrity of the genome) (3-6). H3.3 histone encoded by two genes called H3.3A (H3F3A) and H3.3B (H3F3B) located on chromosome 1 and chromosome 17, respectively. These genes encode an identical histone protein despite the differences in DNA sequence, regulatory region, and promoter sequence (3,5-7). Mutations in these genes lead to some human cancers such as chondroblastoma, osteosarcoma, epithelial ovarian cancer, and pediatric gliomas (3,7,8). One of the most important cancers that diagnosed normally one million people each year in the world is colorectal cancer (CRC) (9,10). CRC is a surface epithelial carcinoma of the colon and rectum caused due to irregular growth of cells and began as clumps of benign cells, called polyps (11). Over time, some of these polyps become cancerous (12). Screening is recommended over the age of 50 or earlier if the patient is at an increased risk of development, to detect the polyps before they become cancerous (13). According to reported of American Cancer Society based on 2015 cancer facts, cancer is caused by environmental factors, such as physical carcinogens, tobacco, infectious organisms, and internal factors, such as genetic changes. Some of these changes like overexpression of epidermal growth factor receptor (EGFR) can cause CRC (14,15). As a result of overexpression of this pathway, some of the transcriptional factors (TFs) such as cAMP response element binding protein (CREB) and activating transcription factor 1 (ATF1) that are H3.3B gene transcription factors, activated (5,14,16).
We assumed that activation of these factors can lead to increased H3.3B gene expression. Therefore, for the first time, we investigated H3.3B gene expression in Iranian CRC patients by relative quantitative real-time polymerase chain reaction (real-time PCR) technique and we would want to study the correlation between the expression of this gene and patient’s demographics and clinicopathological variables.
Methods
RNA extraction and cDNA synthesis
Tumoral and adjacent normal tissues were obtained from 36 patients (normal tissue sampled at least 5 cm from primary tumors) from Hazrat-e-Rasoul Hospital, Tehran, Iran at six years. Tissues specimens immediate snap-freezing in liquid nitrogen and archival at −80 °C until further use. Total RNA was extracted from the CRC samples using a standard protocol with Roche TriPure Isolation Reagent. Quantity and quality of extracted RNA were assessed using spectrophotometer (NanoDrop) and agarose gel electrophoresis, respectively. The homogenization of the extracted total RNA was performed for cDNA synthesis and then, cDNA synthesized with the appropriate amount of RNA at the intended concentration (7,000 ng/µL) of DNA-free total RNA using the Thermo Scientific RevertAid First Strand cDNA synthesis kit according to the manufacturer’s recommendations.
Real-time quantitative PCR
The expression analysis of H3.3B gene was performed by Rotor-Gene 6000 real-time PCR machine using H3.3B and GAPDH specific primers as an interest gene and an endogenous reference gene, respectively (Table 1). Real-time PCR was performed with 0.5 µL of H3.3B cDNA, 0.3 µL of each primer, 5 µL of 2× Yekta-Tajhiz Super SYBR Green qPCR Master Mix in a final volume of 10 µL. PCR conditions were: 95 °C 7 min; then 40 cycles of (95 °C 20 s, 65 °C 12 s, 72 °C 12 s). Real-time PCR raw data analyzed by using Linreg software and PCR replication efficiency and CT numbers were obtained for each reaction, then the expressions fold change of the H3.3B gene were calculated with the Pfaffl mathematical method by using REST 2009 software.

Full table
Patient’s demographics and clinicopathological characteristics
Total of 36 patients included 19 men and 17 women were diagnosed with colorectal cancer. The patient’s demographics (sex and age) and clinicopathological data (tumor localization, historical grading (WHO), histopathological tumor staging, lymph node involvement, and regional lymph node status) of these 36 patients are presented in Table 2.
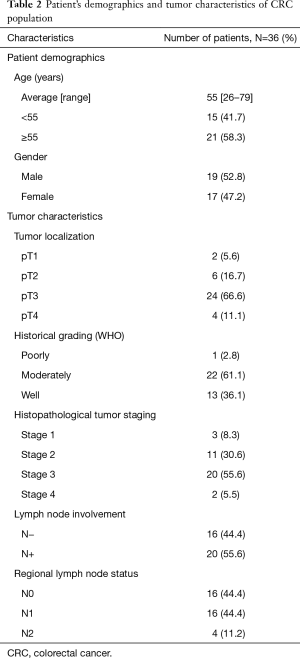
Full table
Statistical analysis of H3.3B gene expression
To determine the methods of statistical analysis, at the first, normality of data was assessed using Kolmogorov-Smirnov, Shapiro-Wilk and other statistical analysis with IBM SPSS Statistics v.24 software. After confirming normal distribution of data, we used independent-samples t-test and one-way ANOVA test to determine the association between expressions of the gene of interest and patient’s demographics and clinicopathological characteristics. In all experiments, 95% confidence interval were used and the P value <0.05 was considered as significant.
Results
Optical density (A260/280) of RNA samples and synthesized cDNA
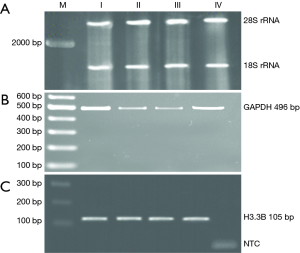
The quantity and quality of extracted RNA were assessed by determining the optical density (OD A260/280) ranging from 1.8 to 2 with the concentration between 3,000 to 8,000 ng/µL and using agarose gel electrophoresis, respectively (Figure 1A). Synthesized cDNA was assessed using GAPDH endogenous reference gene and H3.3B as an interest gene PCR (Figure 1B,C).
Analysis of H3.3B gene expression in normal and tumoral tissues
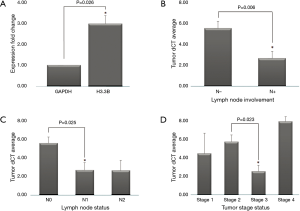
After analysis of real-time PCR raw data and analysis of expression fold change, our results showed that the level of H3.3B gene expression in tumoral tissues is significantly higher than adjacent normal tissues (P value =0.026) (Figure 2A).
Association between H3.3B expression and patient’s demographics and clinicopathological characteristics of the CRC
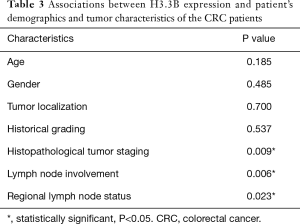
We observed that H3.3B expression was not significantly associated with age, gender, tumor localization, and historical grading but significantly associated with histopathological tumor staging, lymph node involvement, and regional lymph node status of the CRC patients (Table 3). For analysis Post-hoc test, at the first, we assessed equality of variance of significant data by Levene test, then Tukey test was selected. Our finding was shown that H3.3B gene expression, significantly associated with stage 3 (P value =0.023) and N1 (P value =0.025) in histopathological tumor staging and regional lymph node status, respectively (Figure 2B,C,D).
Full table
Discussion
Despite recent advances in prevention, diagnosis, and treatment of cancer, cancer is one of the causes of mortality among people under age 85 (17). Normal cells have a limit cell cycle but cancer cells are able to divide more quickly than normal cells and they are able to exceed the thresholds of the cell cycle (18,19). One of the deadliest cancers in the world based on 2013 cancer facts provided by the American Cancer Society is CRC. According to the International Agency for Research on Cancer the highest and the lowest rate of new cases incidence of CRC, diagnosed in Australia-New Zealand and in Western Africa, respectively (20). There are many genetics mechanisms that can contribute to CRC. One of this mechanism is activation of oncogene pathways (21). CRC caused from 25% to 82% by the increase of EGFR expression in intestinal epithelial cells that can activate Mitogen-activated protein kinases (MAPK) signaling pathway. Overactivation of this pathway promotes cell survival and proliferation factors, regulatory factors, translation and transcription factors such as CREB and ATF1 factors. These two factors are H3.3B gene transcription factors (5,14,16). So, we assumed that increase of these two transcription factors leads to overexpression of H3.3B gene in CRC, the second reason for selected this gene as a candidate gene was, the presence of high amount of H3.3 histone in transcriptionally active regions, promoter regions, and intergenic or intragenic regulatory elements and also the high rate of genes transcription in cancer cells, Furthermore, studies showed that, H3.3 histone can convert silenced chromatin to active chromatin (3-6,22,23). Therefore, we assumed that H3.3B gene that it accumulated in genome active regions and then, can activated gene expression, will be overexpressed in CRC. So for these reasons, we selected this gene as a candidate gene in patients with CRC. In this paper, we demonstrated 3-fold overexpression of H3.3B mRNA in tumoral tissues than adjacent normal tissues. No clear correlation could be observed between H3.3B expression and age, gender, tumor localization, and historical grading but statistical analysis showed that there is the significant association between expressions of this gene and histopathological tumor staging (stage 3), lymph node involvement, and regional lymph node status (N1) of the CRC patients.
Conclusions
Our findings indicate that overexpression of H3.3B gene in stage 3 of CRC can help the doctor to quickly estimate patient’s prognosis of cancer before metastasized, so we can introduce H3.3B gene as a probable prognosis biomarker in patients with CRC.
Acknowledgements
This research was financially supported by the National Institute of Genetic Engineering and Biotechnology, Tehran, Iran.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures followed were approved by the local ethical standards of National Institute of Genetic Engineering and Biotechnology (NIGEB). Written informed consent was obtained from each patient who participated in this study prior to sample collection.
References
- Elsaesser SJ, Goldberg AD, Allis CD. New functions for an old variant: no substitute for histone H3.3. Curr Opin Genet Dev 2010;20:110-7. [Crossref] [PubMed]
- Witt O, Albig W, Doenecke D. Transcriptional regulation of the human replacement histone gene H3.3B. FEBS Lett 1997;408:255-60. [Crossref] [PubMed]
- Yuen BT, Knoepfler PS. Histone H3.3 mutations: a variant path to cancer. Cancer Cell 2013;24:567-74. [Crossref] [PubMed]
- Jang CW, Shibata Y, Starmer J, et al. Histone H3.3 maintains genome integrity during mammalian development. Genes Dev 2015;29:1377-92. [Crossref] [PubMed]
- Frank D, Doenecke D, Albig W. Differential expression of human replacement and cell cycle dependent H3 histone genes. Gene 2003;312:135-43. [Crossref] [PubMed]
- Albig W, Bramlage B, Gruber K, et al. The human replacement histone H3.3B gene (H3F3B). Genomics 1995;30:264-72. [Crossref] [PubMed]
- Behjati S, Tarpey PS, Presneau N, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet 2013;45:1479-82. [Crossref] [PubMed]
- Presneau N, Shen Z, Provencher D, et al. Identification of novel variant, 1484delG in the 3'UTR of H3F3B, a member of the histone 3B replacement family, in ovarian tumors. Int J Oncol 2005;26:1621-7. [PubMed]
- Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet 2010;375:1030-47. [Crossref] [PubMed]
- Tanaka T, Tanaka M, Tanaka T, et al. Biomarkers for colorectal cancer. Int J Mol Sci 2010;11:3209-25. [Crossref] [PubMed]
- Cooper GM. Elements of Human Cancer. Burlington: Jones and Bartlett Publishers, 1992.
- American Cancer Society (ACS). Colorectal Cancer 2014. Available online: http://www.cancer.org/cancer/colonandrectumcancer/detaJGO-16-335iledguide/colon-rectum-cancer-detailed-guide-toc
- Lieberman D. Race, gender, and colorectal cancer screening. Am J Gastroenterol 2005;100:2756-8. [Crossref] [PubMed]
- Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol 2005;6:322-7. [Crossref] [PubMed]
- Krasinskas AM. EGFR Signaling in Colorectal Carcinoma. Patholog Res Int 2011;2011:932932. [Crossref] [PubMed]
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006;58:621-31. [Crossref] [PubMed]
- Alberts D, Hess LM. editors. Fundamentals of Cancer Prevention. Heidelberg: Springer-Verlag, 2008.
- Becker D, Gardner LB. Prevention in clinical practice. Springer Science & Business Media, 2012.
- Kufe DW, Holland JF, Frei E. editors. Holland Frei Cancer Medicine 7, Volume 7. Toronto: BC Decker, 2006.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 2009;361:2449-60. [Crossref] [PubMed]
- Villicaña C, Cruz G, Zurita M. The basal transcription machinery as a target for cancer therapy. Cancer Cell Int 2014;14:18. [Crossref] [PubMed]
- Shi L, Wang J, Hong F, et al. Four amino acids guide the assembly or disassembly of Arabidopsis histone H3.3-containing nucleosomes. Proc Natl Acad Sci U S A 2011;108:10574-8. [Crossref] [PubMed]


