Inflammatory bowel diseases activity in patients undergoing pelvic radiation therapy
Introduction
Inflammatory bowel diseases (IBD), which include Crohn’s disease (CD) and ulcerative colitis (UC), are chronic diseases that generally begin in young adulthood and last throughout life. In the western countries, the incidence and prevalence of IBD has increased in the past 50 years, from 6–15/100,000 to 50–200/100,000 persons/year for CD, and from 8–14/100,000 to 120–200/100,000 persons/year for UC (1). Both CD and UC are complex IBD that result from a combination of genetic predispositions and environmental factors including microbiome in the digestive tract (2,3). Specifically, CD is a transmural inflammatory disease which can affect the entire digestive tract, from the mouth to the anus, and may be complicated by abscesses, fistulas or strictures, whereas UC is limited to the mucosa and can affect only the rectum and colon.
Radical changes have been observed in IBD progression and prognosis with the introduction of steroid therapies in the 1950s, immunosuppressants in the 1970s and more recently biotherapy (4). However, CD and UC remain both a cause of decreased quality of life and nearly half the patients with CD still require at least one surgical resection during disease course (5). These impairments are usually related to periods of active disease with intense severity (6).
Interestingly, different factors promoting IBD activity have been identified. For example, the most established risk factors for flare-ups are prolonged hospitalization, chemotherapy, immunosuppression, hypoalbuminemia, renal insufficiency, use of proton pump inhibitors, while intestinal microbiota dysfunction has been described more recently (7). Although radiation therapy (RT) is commonly considered as one these risk factors, limited evidence is available in the current literature to support such a belief. Indeed, very few retrospective studies and case reports with contradictory results have been published until now. Specifically, Willett et al. observed 28% of severe late gastrointestinal (GI) toxicity (8) while the rate for the same outcome was only 8% in the study by Song et al. (9). Nevertheless some physicians are particularly unwilling to deliver pelvic RT to individuals with IBD, which may dramatically jeopardize the prognosis of those requiring such a treatment alone or as part of a multimodal approach for the management of digestive or uro-gynecological neoplasms.
Against this backdrop of uncertainty regarding the functional impact of RT on IBD, we hypothesized that patients with CD or UC may safely receive pelvic RT with an acceptable toxicity. To achieve our aim, we performed a retrospective analysis of our institutional experience in the management and follow-up of these patients.
Methods
Inclusion criteria
We performed a medical chart review of all consecutive IBD patients treated from 1989 to 2015 by external beam radiation therapy (EBRT) and/or brachytherapy (BT) for a pelvic neoplasm at our academic institution (Gustave Roussy Cancer Campus). Only patients with histologically proven IBD were included in the current study. Schematically, three different types of pelvic neoplasms were considered: digestive, urological or gynecological neoplasms. Clinical staging was performed according to the WHO, TNM and D’Amico classification for anal, rectal and prostate cancers, respectively, whereas the FIGO staging system was used for cervix, and endometrium cancers.
Treatment
The different RT modalities considered for the purpose of this study were EBRT using conventional 2D, conformal 3D, or intensity-modulated RT (IMRT) techniques as well as BT using high-dose rate (HDR), low-dose rate (LDR) or pulse dose rate (PDR) techniques. Regardless of the technique, RT was indicated either as the primary treatment of the aforementioned pelvic tumors or as a perioperative option before or after radical surgery. EBRT and BT were used mostly alone or occasionally in combination. The use of systemic or androgen deprivation therapies during the treatment was recorded as well. All treatments were discussed at a multidisciplinary tumor board.
Follow-up
During the treatment period, patients were followed at least once week by a radiation oncologist to detect any acute toxicity related to RT and referred to a gastroenterologist in case of IBD flare-up. In addition to the usual clinical aspects examined during the treatment period, IBD activity and GI toxicity was closely monitored. Specifically, the severity of CD and UC was assessed using the Harvey-Bradshaw (HB) and Mayo index, respectively (10,11). IBD activity was considered as (I) severe if HB index ≥16 for CD or Mayo index ≥7 for UC; (II) moderate if 8≤ HB index ≤15 for CD or 5≤ Mayo index ≤6 for UC; (III) mild if 5≤ HB index ≤7 for CD or 2≤ Mayo index ≤4 for UC. Patients were considered in remission when HB index ≤4 for CD or Mayo index ≤1 for UC. The GI toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) of The National Cancer Institute of the National Institutes of Health. If scores weren’t assessed at baseline (notably for patients treated in the 80-ies or 90-ies) they were estimated retrospectively according to their specific components.
Long-term follow up was performed alternatively by both radiation oncologist and gastroenterologist. Patients were regularly evaluated with a specific focus on IBD activity using also HB and Mayo index for CD and UC, respectively.
End points
The primary endpoint of our study was to examine the functional impact of RT on IBD activity by using HB and Mayo indices during and after the treatment period. As such, both early and long term IBD activity were considered.
Secondary endpoints included other functional outcomes such as GI toxicity graded according to the CTCAE as well as oncological outcomes including locoregional recurrence-free survival (LRRFS) defined as the time from RT start to any local and/or pelvic failure and overall survival (OS) defined as the time from RT start to death from any cause.
Statistical analysis
Continuous variables were reported as median with corresponding interquartile range (IQR), whereas frequencies and proportions were used for categorical variables. We performed a narrative description of early and long term IBD activity as well as digestive tolerance and oncological outcomes in patients undergoing pelvic RT.
In addition, we tested the correlation between several qualitative or quantitative variables and the rank sum of (I) maximum IBD activity as well as (II) maximum GI toxicity, both within and after 6 months from the beginning of RT. The Mann-Whitney U test was used for the qualitative variables which included (I) patient and IBD-related characteristics such as tobacco smoking (absent or present), IBD type (CD or UC) and IBD location (absence or presence of rectum, colon, or small intestine location); (II) tumor-related characteristics such as primary neoplasm type (absence or presence of prostate, cervix, endometrium, rectum or anal cancers); (III) treatment-related characteristics such as RT modality (absence or presence of EBRT or BCT), EBRT modality (absence or presence of 2D or 3D), combination of EBRT with BT (absent or present), pelvic lymph node irradiation (absent or present), prior pelvic surgery (absent or present), and systemic chemotherapy (absent or present). Alternatively, the Pearson product-moment correlation coefficient was used for the quantitative variables, which included (I) patient and IBD-related characteristics such as baseline IBD activity, and body mass index (BMI); (II) treatment-related characteristics such as interval between IBD diagnosis and RT, EBRT dose and the number of EBRT fields.
Finally, we performed survival analyses by using the Kaplan-Meier method to estimate LRRFS and OS.
For all analyses, a two-side P value <0.05 was considered significant. Statistical analyses were performed using SigmaPlot 12.5 software.
Results
Patients and tumors
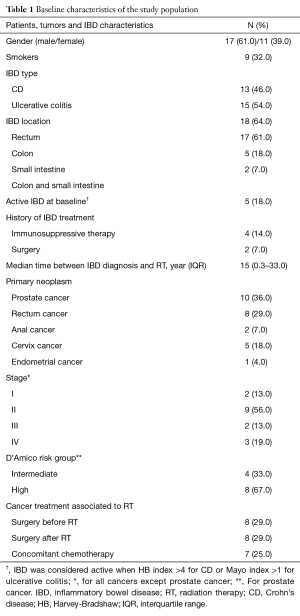
A total of 28 IBD patients who received RT for prostate cancer (n=12), rectum cancer (n=8), cervix cancer (n=5), anal cancer (n=2) and endometrial cancer (n=1) were included. Baseline clinical and pathological characteristics are reported in Table 1. There were four intermediate and eight high-risk prostate cancers. Regarding other neoplasms, distribution of clinical stage was as follows: stage I (n=2), stage II (n=9), stage 3 (n=2), stage IV (n=3).
Full table
In the population study, 13 (46%) and 15 (54%) patients had CD and UC, respectively. Only 2 (7%) with UC and 3 (11%) with CD patients had active IBD at the time of RT, whereas 21 (75%) required specific IBD medication including steroids, azathioprine or biotherapy. The median follow-up was 5.9 years (IQR, 0.5–24 years).
Cancer treatment
Overall, 18 (64%) patients received pelvic EBRT alone. A median dose of 53 Gy (IQR, 33.6–74 Gy) was delivered to the pelvis with a median dose per fraction of 2 Gy (IQR, 1.8–5 Gy). Conventional (2D) and conformal (3D) techniques were used in 17 (61%) and 8 (29%) patients whereas one (3.5%) patient received IMRT.
Only 2 (7.1%) patients received BT alone whereas it was delivered concomitantly with EBRT in 8 (3.5%) patients. A median dose of 15 Gy (IQR, 12–60 Gy) was delivered to the pelvis. HDR and LDR techniques were used in 2 (7.1%) and 5 (17.9%) patients, respectively, whereas 2 (7.1%) patients received PDR. One (3.5%) patient received permanent iodine 125 BT.
A concomitant systemic chemotherapy was delivered in 7 (25%) patients. Specifically, the regimen was cisplatin, capecitabine or 5-fluorouracil (5FU) alone in 3 (10.7%), 1 (3.5%) or 1 (3.5%) patients, respectively, whereas combination of cisplatin or oxaliplatin plus 5FU was used for 1 (3.5%) or 1 (3.5%) patient, respectively. In addition, 4 (14.3%) patients with prostate cancer received androgen deprivation therapy during RT.
Finally, surgery was performed in 16 (5.9%) patients, who received RT as a neoadjuvant (n=8; 28.6%) or adjuvant therapy (n=8, 28.6%).
IBD activity
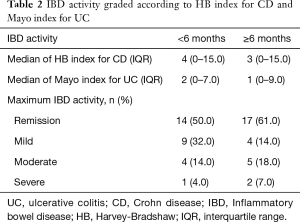
The median of HB and Mayo index for CD and UC patients within 6 months of follow-up after RT was 4 (IQR, 0–15) and 2 (IQR, 0–7), respectively. During this time period, 1 (3.5%) prostate cancer patient experienced a severe UC activity after a total dose of 74 Gy (up to 46 Gy to the seminal vesicles) delivered using EBRT, while 14 (50%) patients were in remission.
The median of HB and Mayo index for CD and UC patients from 6 months to last follow-up after RT was 3 (IQR, 0–15) and 1 (IQR, 0–9), respectively. During this time period, 2 (7.1%) prostate cancer patients experienced a severe UC activity including 1 (3.5%) who received a total dose of 74 Gy (up to 46 Gy to the seminal vesicles) delivered using EBRT, it was the same patient who had a severe flare up within 6 months of follow-up, and 1 (3.5%) who received a total dose of 46 Gy to the prostate and vesicle seminal delivered using EBRT associated to a 14 Gy prostate boost delivered using BT with HDR technique. It is noteworthy that none of them had an active IBD at baseline. Conversely, 17 (60.7%) patients were in remission. Scores of acute and late IBD activity are summarized in Table 2.
Full table
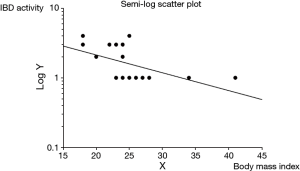
In addition, only rectal IBD location (P=0.012) was significantly correlated with an increased maximum IBD activity within 6 months of follow-up, whereas only decreased BMI (P=0.016) was significantly correlated with an increased maximum IBD activity after 6 months of follow-up. Figure 1 shows the semi log scatter plot of IBD activity after 6 months of follow-up vs. BMI. No other tested characteristic was significantly correlated with IBD activity.
GI toxicity
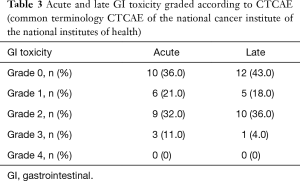
Grade 3 acute GI toxicities were observed in 3 (11%) patients who were admitted for massive diarrhea, while no grade 4 acute toxicity was reported. Grade 3 late GI toxicity was observed in 1 (3.5%) patient (small bowel obstruction), while no grade 4 late grade toxicity was reported. Acute and late GI toxicities are summarized in Table 3.
Full table
No patient-, IBD-, tumor- or treatment-related characteristic was significantly correlated to the maximum GI toxicity within or after 6 months of follow-up.
Oncological outcomes
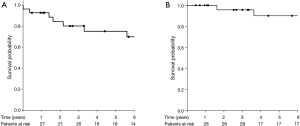
During the follow-up period, 11 (39%) patients experienced tumor recurrence. The median time to recurrence was 25.2 months (IQR, 2–174 months). Loco-regional and metastatic recurrences occurred in 16 (58%) and 4 (14%) patients, respectively. Loco-regional and distant recurrences were noted in 2 (7.1%) patients, respectively. The 5-year LRRFS rate was 75% (95% CI, 60–95%) (Figure 2A). During the follow-up period, 7 (25%) patients died, including 5 (18%) from the disease. The 5-year OS rate was 90% (95% CI, 78–100%) (Figure 2B).
Discussion
Although little data is available on the tolerance of RT in IBD patients, CD and UC currently represent both a significant limit to deliver such a treatment for a large part of the radiation oncologists’ community. This may be based on physiopathological studies suggesting a potential adverse effect of the free radicals from chronic IBD inflammation (12), (I) the deficiency in DNA repair of IBD patients (13); and (II) the IBD related vasculitis, which could contribute to the late toxicity of RT (14). Nonetheless, this excessive concern for potential risk of uncontrolled flare-ups and GI toxicity may lead to the use of alternative treatment modalities, which could potentially jeopardize the prognosis of individuals requiring RT in their treatment sequence.
Interestingly, we found in the present study that RT was well tolerated by IBD patients. Specifically, for a median delivered dose of 53 Gy, HB and Mayo indices remained generally low during the median follow-up period of 5.9 years and only 1 (3.6%) and 2 (7.1%) prostate cancer patients experienced severe IBD activity within and after 6 months following RT, respectively. In addition, more than 60% of the study population was considered in IBD remission after 6 months of follow-up. Similarly, GI toxicity was acceptable with grade 3/4 acute and late GI toxicities occurring in only 3 (11%) and 1 (3.6%) patients.
To our knowledge only five retrospective studies including more than 15 IBD patients who received pelvic RT have been published until now (6,9,15-17). It is noteworthy that these reports mostly described GI toxicity related to the treatment but no focus was made on IBD activity by using standardized scores as HB and Mayo index. As such, despite the low number of cases, our study represents the largest dedicated assessment of IBD activity in patients undergoing RT for pelvic malignancies.
Interestingly, we found lower rates of early and late GI toxicity, when compared to the aforementioned limited evidence available in the literature. Indeed, Willett et al. reported 21% and 28% of grade 3/4 acute and late GI toxicity respectively, in a series of 28 IBD patients with CD (n=10), or UC (n=18) (6). In that study, the mean dose delivered to the pelvis was 40 Gy and 51.1 Gy for conventional and specialized technique, respectively, and the authors observed a decreased risk of late GI toxicity from 73% to 23% in patients treated with specialized techniques (small fields, decubitus position, proton beam irradiation, or scheduled rest periods during treatment) or surgical procedures (clips to delineate tumor bed, omentoplasty, or Dexon Mesh) to minimize small intestine and colon irradiation. The same rate of grade 3/4 acute GI toxicity (20%) without any late side effects was found in the study by Green et al. who analyzed 15 IBD patients with rectal cancer including 13 patients treated by either neoadjuvant or adjuvant RT ± 5FU (15). The median dose of EBRT delivered to the pelvis was 50.4 Gy. Furthermore, Song et al. identified 17 IBD patients who received a median dose of 45 Gy of RT delivered to the pelvis or abdomen with (n=15) or without (n=2) concomitant systemic chemotherapy and they described 21% and 8% of grade 3/4 acute and late GI toxicity, respectively (9). Finally, GI toxicity of BT in IBD patients was assessed in the study by Peters et al., who reported only mild to moderate side effects without any grade 3/4 GI toxicity in a population of men with prostate cancer (16). As such, the inclusion of patients treated with BT may have contributed to the lower rates of acute and late GI toxicity in our study. Indeed, several reports suggested that such a RT technique could be safer in IBD patients, notably by reducing the dose delivered to the small intestine (16,18,19). It is noteworthy that none of these studies have reported any death from GI toxicity.
The selection of IBD patients fit to receive RT is also highly challenging. We found that the IBD location could be a determinant factor to predict evolution of IBD activity after RT, with a significant increased maximum IBD activity within 6 months after RT for patients with a rectal IBD location. Although the role of patient’s weight in the evolution of IBD is widely debated, our results suggest also a significant correlation between low BMI and high IBD activity after 6 months of follow-up. However, only 24 (86%) were included in this analysis, as data were missing for 4 (14%).
Regarding oncological outcomes, the estimated 5-year OS and LRRFS rates in our study were 90% and 75%, respectively. These favorable outcomes are consistent with other studies suggesting that providing RT could avoid jeopardizing IBD patient prognosis. Indeed, Green et al. observed in a population of IBD patients with rectal cancer, a trend toward improved pelvic control in those who received EBRT with a 5-year pelvic control rate of 60% vs. 23% (15). However, our oncological outcomes are based on heterogeneous data from patients with different pelvic malignancies, which makes difficult to draw any definitive conclusion.
Because the majority of patients included in our study were treated with 2D techniques, no dosimetric data regarding bowel exposure can be analyzed. However technological advances in RT should help to reduce the toxicities in such patients. Indeed White et al. (17) found that the use of IMRT was associated with decreased acute bowel toxicity in this population.
Other limitations of the present study include the retrospective design and the low number of included patients limiting analysis capabilities.
Conclusions
To summarize, we report an acceptable tolerance of RT in IBD patients with pelvic malignancies. Specifically, a low risk of uncontrolled flare-up was observed during the follow-up period and acute or late GI toxicity were moderate. Only patients with rectal IBD localization or low BMI may experience more severe IBD activity within or after 6 months following RT, respectively. Other studies should focus on determining which RT technique could be used to minimize even more RT toxicity in IBD patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The data analyzed were retrospectively collected. All patient treatments were approved by a tumor board review following the national guidelines. In accordance with European regulation, French observational studies from data obtained without any additional therapy or monitoring procedure, do not need the approval of a Review Board/Independent Ethics Committee.
References
- Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785-94. [Crossref] [PubMed]
- Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut 2013;62:1505-10. [Crossref] [PubMed]
- Jianzhong H. The genetic predisposition and the interplay of host genetics and gut microbiome in Crohn disease. Clin Lab Med 2014;34:763-70. [Crossref] [PubMed]
- Annese V, Duricova D, Gower-Rousseau C, et al. Impact of New Treatments on Hospitalisation, Surgery, Infection, and Mortality in IBD: a Focus Paper by the Epidemiology Committee of ECCO. J Crohns Colitis 2016;10:216-25. [Crossref] [PubMed]
- Lopez J, Konijeti GG, Nguyen DD, et al. Natural history of Crohn's disease following total colectomy and end ileostomy. Inflamm Bowel Dis 2014;20:1236-41. [Crossref] [PubMed]
- Lönnfors S, Vermeire S, Greco M, et al. IBD and health-related quality of life -- discovering the true impact. J Crohns Colitis 2014;8:1281-6. [Crossref] [PubMed]
- Sobczak M, Fabisiak A, Murawska N, et al. Current overview of extrinsic and intrinsic factors in etiology and progression of inflammatory bowel diseases. Pharmacol Rep 2014;66:766-75. [Crossref] [PubMed]
- Willett CG, Ooi CJ, Zietman AL, et al. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys 2000;46:995-8. [Crossref] [PubMed]
- Song DY, Lawrie WT, Abrams RA, et al. Acute and late radiotherapy toxicity in patients with inflammatory bowel disease. Int J Radiat Oncol Biol Phys 2001;51:455-9. [Crossref] [PubMed]
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625-9. [Crossref] [PubMed]
- Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [Crossref] [PubMed]
- Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet 1994;344:859-61. [Crossref] [PubMed]
- Sanford KK, Price FM, Brodeur C, et al. Deficient DNA repair in chronic ulcerative colitis. Cancer Detect Prev 1997;21:540-5. [PubMed]
- Korzenik IBD JR. A Vascular Disorder? The Case for Heparin Therapy. Inflamm Bowel Dis 1997;3:87-94. [PubMed]
- Green S, Stock RG, Greenstein AJ. Rectal cancer and inflammatory bowel disease: natural history and implications for radiation therapy. Int J Radiat Oncol Biol Phys 1999;44:835-40. [Crossref] [PubMed]
- Peters CA, Cesaretti JA, Stone NN, et al. Low-dose rate prostate brachytherapy is well tolerated in patients with a history of inflammatory bowel disease. Int J Radiat Oncol Biol Phys 2006;66:424-9. [Crossref] [PubMed]
- White EC, Murphy JD, Chang DT, et al. Low Toxicity in Inflammatory Bowel Disease Patients Treated With Abdominal and Pelvic Radiation Therapy. Am J Clin Oncol 2015;38:564-9. [Crossref] [PubMed]
- Kapp KS, Stuecklschweiger GF, Kapp DS, et al. Carcinoma of the cervix: analysis of complications after primary external beam radiation and Ir-192 HDR brachytherapy. Radiother Oncol 1997;42:143-53. [Crossref] [PubMed]
- Tromp D, Christie DRH. Acute and Late Bowel Toxicity in Radiotherapy Patients with Inflammatory Bowel Disease: A Systematic Review. Clin Oncol (R Coll Radiol) 2015;27:536-41. [Crossref] [PubMed]