High dose chemoradiation for unresectable hilar cholangiocarcinomas using intensity modulated external beam radiotherapy: a single tertiary care centre experience
Introduction
Cholangiocarcinomas (CCA) are rare tumours of biliary tract. They are classified as intra hepatic or extrahepatic depending on the location of the tumour. Hilar CCA (also known as Klatskin’s tumour) arises at the confluence of right and left bile ducts and are often locally advanced at presentation with perihilar infiltration (1). Only 20–30% of these patients are amenable to upfront curative resection. Surgery is often extensive involving hepatic resection for achieving negative margins (2-6). Tumors that are not amenable to curative surgical resection are generally treated with either palliative chemotherapy alone using Gemcitabine and Cisplatin or combined chemoradiation (7,8) after biliary drainage. Addition of radiation is shown to prolong the patency of stent and also improve survival (9).
Radiation therapy can be delivered by either external beam radiotherapy (EBRT) or endobiliary brachytherapy (EBBT) or combination of both techniques. The advent of intensity modulated radiotherapy (IMRT) an EBRT mode today, facilitates the delivery of high radiation doses to several upper gastrointestinal tumours with acceptable grade III–IV morbidity (10).
We present the retrospective analysis of a prospectively maintained database where high dose radiation was delivered to non-metastatic locally advanced hilar CCA which were deemed unresectable at a tertiary referral cancer centre. They were treated with chemotherapy combined with high dose radiation using either EBRT alone or sequentially after brachytherapy. Our aim was to compare the outcomes of these two modalities of high dose radiation delivery.
Methods
All patients without any evidence of distant metastasis who were deemed unresectable by the hepatobiliary surgeons due to locally advanced disease were eligible for the study. Locally advanced tumors were considered unresectable when the mass was found to involve both the hepatic ducts with bilateral parenchymal extension or tumors having bilateral vascular involvement at surgical exploration or at preoperative staging. An informed consent was taken from all the patients.
Patients presenting to us with obstructive jaundice were planned for intraluminal brachytherapy followed EBRT (EBBT + EBRT, group 1). In these patients, percutaneous biliary drainage (PTBD) was selected as the route for biliary drainage since it facilitates mapping of proximal extent of spread of the tumor into the biliary tree, tissue sampling to confirm the diagnosis and also placement of intraluminal brachytherapy catheters. Patients who had already undergone biliary drainage prior to registration at or institute and were fit for treatment were selected for EBRT with chemotherapy (group 2).
Patients with ECOG performance status of 2 or less and no co-morbidity precluding treatment were considered fit for combined chemoradiation once the serum bilirubin was less than 3 mg/dL and in the absence of cholangitis.
Both groups were planned to be treated with high dose radiation in biologically equipotent doses.
Group 1 was planned to be initially treated with brachytherapy alone followed by EBRT along with chemotherapy. Brachytherapy was delivered through the internal-external PTBD drainage catheters over consecutive 3 days. Post endobiliary radiation the catheters were then replaced by bilateral metal stents and patients were discharged from the hospital. Four to six weeks following the metal stenting, patients were clinically assessed for fitness for EBRT along with concomitant chemotherapy. They were reimaged using a combination of Positron emission Tomography with Contrast Enhanced Computed Tomography (PET-CT). If the imaging did not reveal any evidence of distant metastasis and there was no cholangitis, patients were administered concurrent chemotherapy with EBRT at a dose fractionation of 45 Gy/25 fractions/5 weeks. The treatment protocol in group 2 was concurrent chemoradiation using EBRT alone, delivered in dose of 55–57 Gy/25 fractions/5 weeks along chemotherapy.
Brachytherapy procedure
Magnetic resonance cholangiopancreatography (MRCP), MRI and contrast enhanced CT scan images were reviewed to classify the block by Bismuths classification. The extent and length of the stricture and its proximal limit was defined during percutaneous transhepatic cholangiogram (PTC gram) performed at the time of biliary drainage. Once the post PTBD complications if any abated, brachytherapy catheters (one each in right and left ductal system) were inserted through the internal external PTBD catheters and positioned across the stricture. Care was taken to position the brachytherapy catheter under fluoroscopic guidance. Contrast enhanced CT images were obtained at inter-slice distance of 2 mm and exported to PLATO (v.14. 3, Nucletron B.V, an Elekta company, Elekta AB, Stockholm, Sweden) planning system. After planning, treatment was delivered using iridium-192 high-dose-rate BT to a dose of 3–4 Gy/fraction/two fractions per day for total of 4 fractions thus delivering a total dose of 14 Gy. The brachytherapy dose was prescribed at the target volume delineated on the planning CT scans. After completion of EBBT, the catheters were removed and metallic stent was placed across the stricture the following day.
EBRT procedure
External beam radiotherapy simulation and planning was done and tumor was delineated on the CT planning images. If the lesion was poorly visualized on diagnostic imaging additional PET-CT based planning was performed. Gross tumour volume (GTV) was delineated; 5 mm to 1 cm margin was given to GTV to delineate Clinical target volume (CTV). Planning target volume (PTV) was generated by giving 1.5 cm expansion in supero-inferior direction and 0.8 cm expansion in other directions. Adjacent lymph nodes like peri portal nodes if detected were included in the CTV. All patients were treated with image guided intensity modulated RT (IG-IMRT) using cone beam CT. Daily image guidance was done prior to treatment delivery.
Radiotherapy dose modulation
The EBRT dose schedules were planned with an aim to achieve similar radiobiologically effective doses in both groups of patients. The EBRT plans were optimized to keep doses to liver, duodenum and small bowel within tolerance limits (30% of liver receives less than 30 Gy, the volume of duodenum receiving 50 Gy was limited to 25 cc).
Chemotherapy protocols
The chemotherapy schedule administered was similar in both groups. Intravenous Gemcitabine (300 mg/m2) was administered as a short infusion over 30 minutes once every week for 5 weeks consecutively along with radiation. Systemic chemotherapy was considered for those with progressive disease detected at response evaluation done at 6 weeks or during follow up. A combination of Gemcitabine and Oxaliplatin was used. It was administered as Gemcitabine 1,000 mg/m2 as a 10-mg/m2/min infusion on day 1 and Oxaliplatin 100 mg/m2 as a 2-hour infusion on day 2 every 2 weeks.
Follow up
After completion of treatment, all patients were assessed for response at 6 weeks. Imaging protocol included a CT scan of abdomen and pelvis alone for disease that was clearly visible at baseline. In those patients where baseline CT scan of MRI did not reveal clear mass forming lesions patients were assessed by the combined PET-CT scan. In addition, Serum CA19-9 was ordered. In patients on systemic therapy, CT scan was performed to assess response after every four cycles. Toxicities, both acute and late were recorded as per CTCAE v3.0 grading system.
Statistical analysis
All statistical analysis was done using Statistical Package for the Social Sciences (SPSS 17) software. Survival analysis was done using Kaplan Meier methods and curves were obtained. Univariate analysis for prognostic factors was done with Log-rank test.
Results
Between August 2005 to December 2012, 68 consecutively treated patients were analyzed. The median age of the cohort was 52 years (range, 45–60 years) with male predominance (59%). The mean and median serum bilirubin level at baseline was 11.6 and 6.8 mg/dL respectively. The median baseline CA 19-9 level was 330 U/mL. Histopathological diagnosis was available in 54 (79%) patients (Table 1). Despite multiple attempts (maximum of four) at biliary brushings performed at PTBD no histological confirmation could be obtained in 14 (21%) patients. These patients were treated based on radiological evidence of cholangiocarcinoma. Seven patients did not have well defined mass lesion but had positive histology.
Full table
Fifty (73%) patients on Group 1 who presented with obstructive jaundice were drained by PTBD. There were PTBD related complications. All these 50 patients received brachytherapy as per protocol. The median dose of EBBT administered was 14 Gy (range, 14–20 Gy) HDR. Twenty one patients could not receive further EBRT due to development of recurrent cholangitis with liver failure in 11, distant metastasis on reimaging scans done at 6 weeks in 7 and refusal of further treatment in 3 patients. Thus, only 29 (43%) could undergo further treatment with EBRT as per protocol. The median dose of EBRT administered following EBBT was 45 Gy (range, 40–5 Gy). Concurrent chemotherapy with Inj. Gemcitabine as per protocol was given to all the patients receiving EBRT. The median number of chemotherapy cycles was 5 (range, 3–6).
In all eighteen (27%) patients in group 2 were treated with concurrent chemotherapy and high dose EBRT alone at a median dose of 57 Gy (range, 50–57 Gy) over 25 fractions as per the planned protocol. The median number of chemotherapy cycles was 5 (range, 3–6).
Thus 47 patients (both group 1 & 2) who completed the planned protocol were evaluated for response at 6 weeks. In addition to CECT, PET scans was used as a marker of response in 28 (59%) patients in whom baseline PET-CT was performed as planned. The response rates did not significantly differ between the two groups. Twenty-six (55%) patients achieved complete radiological response, 16 (64%) belonging to group 1 and 8 (44%) of group 2 P=0.05 (Table 2).

Full table
Complications
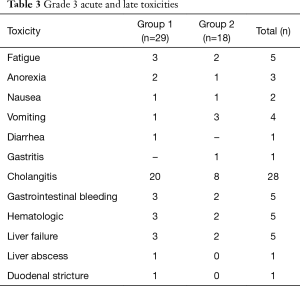
None of patients had complication during insertion or removal of BT catheters. The main acute toxicities were anorexia, fatigue and nausea and were managed conservatively (Table 3). Four patients had late radiation toxicity, of these three had hematemesis due to duodenitis and one had RT induced stricture at gastro-esophageal junction requiring dilatation. There were no deaths related to late radiation induced effects. Late cholangitis occurred in twenty-eight patients due to stent block of which eight patients succumbed to it.
Full table
Survival outcomes
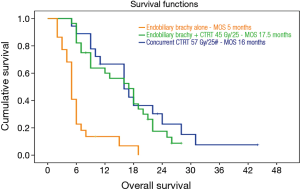
At the median follow up 12 months (range, 6–19 months) the median overall survival (MOS) for the 47 (69%) patients who completed the planned protocol was similar in both groups. It was 17.5 months for group 1 and 16 months for group 2 (P=0.38). In contrast, there was a distinctly poor MOS of 5 months only amongst those 21 patients who failed to complete the planned concomitant CTRT after completion of EBBT (Figure 1). The OS for group1 was 65% and 18% at 1 and 2 years and 57% and 24% for group 2.
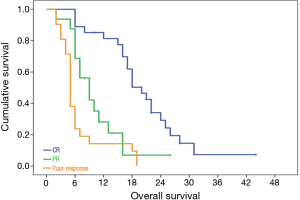
Primary tumor response to radiotherapy at 6 weeks, had direct bearing on overall survival. The median OS was 20 months for patients having complete response, 9 months for partial and 5 months for the remaining poor responders P=0.001.
Thus the most important factor dictating improved OS beyond 2 years was the complete response of the primary tumor to radiotherapy, regardless of the protocol which was used. A total of 10 patients, 6 in group 1 and 4 in group 2 survived beyond 2 years (Figure 2).
The main sites of distant disease progression were peritoneum (13 patients) and liver (4 patients). Other sites of distant metastasis were abdominal wall, neck nodes and brain.
Discussion
Surgery remains the mainstay of treatment for CCA. The median OS post curative resection ranges from 20 months to 48 months in various series (5,6). However, complete surgical resection of hilar CCA can be done only in few patients. In a study reported by Isayama et al., OS was significantly better for patients undergoing curative resection as compared to RT or best supportive care (BSC) with median survival of 48.7 months in the surgery group, 22.1 months in the RT group, and 5.7 months in the BSC group (9), demonstrating that RT remains an important treatment modality for patients with unresectable hilar CCA. In our study, the median survival of 17 months achieved for the patients receiving radiotherapy is comparable.
Various studies have shown post radiation outcomes to be significantly better as compared to endobiliary stenting alone (11). A single small randomized study reported by Válek et al. (11) showed that the MOS was 298 vs. 387.9 days (P=0.05) in those treated with biliary stent alone v/s biliary stent followed by EBBT and EBRT respectively. Further, increasing the doses of RT have shown to improve both PFS and MOS. Crane et al observed that time to local progression was prolonged in the patients receiving RT doses between 54 to 85 Gy (12). This dose escalation can be achieved using EBRT alone or a combination of EBBT and EBRT (13-16). Our study supports this view as we found no difference in MOS between patients in group I and group 2, indicating that it is high dose radiation which is effective regardless of the mode of delivery. In patients in whom brachytherapy is not feasible, equipotent dose can be delivered be safely by with higher doses of EBRT using modern radiotherapy techniques. Alden et al., reported that combination of chemoradiation and brachytherapy with doses more than 55 Gy achieved a median survival of 24 months as compared to only 6 months when doses below 55 Gy were used (P=0.0003). A lengthening of the median survival with increasing radiation dose (4.5, 9, 18 and 25 months for <45, 45–55, 55–65, 66–70 Gy, respectively) was reported in another study (14). The MOS of 17.5 and 16 months achieved in group 1 and 2 respectively was similar to the studies delivering similar doses of radiation.
EBBT alone is also shown to prolong the stent patency rate (17). In practice, several patients may fail to be subsequently fit for EBRT or palliative chemotherapy after biliary stenting. Since EBBT can delivered at the time of PTBD, it may be used to prolong the stent patency.
Radiological assessment of response can be challenging in hilar cholangiocarcinoma, particularly in those with a diffuse/infiltrative disease pattern. In this study patient having complete radiological regression of tumors had significantly better survival. Therefore all attempts must be made to image and map these tumors with multiple imaging modalities primarily for drawing accurate target volumes for RT planning, to assess response and plan further management.
The late toxicity in terms of duodenal and gastric ulcers is a matter of concern when using higher radiation doses. In some studies the incidence ranges from 9% to 40% (16,18,19). Since this depends on the proximity of the tumor to the duodenal and gastric mucosa, modern techniques using IMRT with IGRT can be potentially applied to reduce these late complications. In our series where IMRT and IGRT were used for all patients, only 3 (6%) developed duodenal ulcers.
The strength of present series includes prospectively maintained data base, uniform patient selection and treatment protocols. However, it has few limitations like retrospective analysis, small number of patients and impact of chemotherapy on MOS when used after progression.
In conclusion, our study indicates that the best chance of local control and survival in unresectable Klatskin tumors can be achieved by delivering higher doses of Radiation of >45 Gy. Higher radiation doses can either be delivered by EBRT or a combination of EBRT and a boost brachytherapy preferably using IMRT. This approach is relatively safe when dose escalated radiation is delivered using modern radiotherapy techniques in order to minimize toxicity and improve survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This is a retrospective analysis of the prospectively maintained data of the routine standard treatment offered to our patients, hence no formal approval from the ethics committee was taken. However, informed consent was taken from all the patients for the treatment.
References
- Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. an unusual tumor with distinctive clinical and pathological features. Am J Med 1965;38:241-56. [Crossref] [PubMed]
- Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31-8. [Crossref] [PubMed]
- Inouye AA, Whelan TJ Jr. Carcinoma of the extrahepatic bile ducts: a ten year experience in Hawaii. Am J Surg 1978;136:90-5. [Crossref] [PubMed]
- Blumgart LH, Hadjis NS, Benjamin IS, et al. Surgical approaches to cholangiocarcinoma at confluence of hepatic ducts. Lancet 1984;1:66-70. [Crossref] [PubMed]
- Langer JC, Langer B, Taylor BR, et al. Carcinoma of the extrahepatic bile ducts: results of an aggressive surgical approach. Surgery 1985;98:752-9. [PubMed]
- Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg 2003;238:73-83. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Morganti AG, Trodella L, Valentini V, et al. Combined modality treatment in unresectable extrahepatic biliary carcinoma. Int J Radiat Oncol Biol Phys 2000;46:913-9. [Crossref] [PubMed]
- Isayama H, Tsujino T, Nakai Y, et al. Clinical benefit of radiation therapy and metallic stenting for unresectable hilar cholangiocarcinoma. World J Gastroenterol 2012;18:2364-70. [Crossref] [PubMed]
- Polistina FA, Guglielmi R, Baiocchi C, et al. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother Oncol 2011;99:120-3. [Crossref] [PubMed]
- Válek V, Kysela P, Kala Z, et al. Brachytherapy and percutaneous stenting in the treatment of cholangiocarcinoma: a prospective randomised study. Eur J Radiol 2007;62:175-9. [Crossref] [PubMed]
- Crane CH, Macdonald KO, Vauthey JN, et al. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys 2002;53:969-74. [Crossref] [PubMed]
- Shin HS, Seong J, Kim WC, et al. Combination of external beam irradiation and high-dose-rate intraluminal brachytherapy for inoperable carcinoma of the extrahepatic bile ducts. Int J Radiat Oncol Biol Phys 2003;57:105-12. [Crossref] [PubMed]
- Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys 1994;28:945-51. [Crossref] [PubMed]
- Ghafoori AP, Nelson JW, Willett CG, et al. Radiotherapy in the treatment of patients with unresectable extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2011;81:654-9. [Crossref] [PubMed]
- Deodato F, Clemente G, Mattiucci GC, et al. Chemoradiation and brachytherapy in biliary tract carcinoma: long-term results. Int J Radiat Oncol Biol Phys 2006;64:483-8. [Crossref] [PubMed]
- Chen Y, Wang XL, Yan ZP, et al. HDR-192Ir intraluminal brachytherapy in treatment of malignant obstructive jaundice. World J Gastroenterol 2004;10:3506-10. [Crossref] [PubMed]
- Wagner HJ, Knyrim K, Vakil N, et al. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy 1993;25:213-8. [Crossref] [PubMed]
- Foo ML, Gunderson LL, Bender CE, et al. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys 1997;39:929-35. [Crossref] [PubMed]