Effect of Helicobacter pylori infection on outcomes in resected gastric and gastroesophageal junction cancer
Introduction
Gastric cancer is the 5th most common cancer worldwide and kills approximately 723,000 people every year (1). Helicobacter pylori (H pylori) infection has been associated with an up to six-fold increase in risk of developing gastric cancer (GC), but its relationship with gastroesophageal junction (GEJ) cancer is less well defined (2-5). H pylori causes chronic active inflammation of the gastric mucosa leading to loss of gastric glands (atrophic gastritis), which progresses to intestinal metaplasia and dysplasia, and adenocarcinoma (6,7). H pylori is typically seen in developing countries and has been associated with poorer socioeconomic status (8,9). Eradication therapy for H pylori has been tied to lower rates of lower rates of GC (10,11), however it role may be more limited once histologic intestinal metaplasia or severe atrophy develops (12).
Studies evaluating the impact of H pylori infection on outcomes in early stage GC are limited. The purpose of this study was to evaluate the differences in clinical-pathologic features and outcomes of patients with GC and GEJ cancer based on H pylori status.
Methods
We retrospectively reviewed the medical records of patients over the age of 18 years who underwent curative resection for GC and GEJ cancer at Mount Sinai Hospital between 2007 and 2012. All patients had histopathologic documentation of the presence or absence of H pylori infection. Eligible patients were identified via an institutional database and tumor registry using ICD diagnosis codes. Study data was collected from patients’ medical records.
Patients with a prior curatively treated non gastric, non gastroesophageal junction malignancy were included, but those who developed a secondary malignancy post GC/GEJ cancer diagnosis were excluded.
Demographic, clinical, pathologic, treatment and outcomes information was collected. Histologic evaluation was performed according to the Lauren classification (intestinal, diffuse, mixed) and histologic type (well, moderately, or poorly differentiated, signet ring cell) (13). The standard surgical treatment was radical total or subtotal gastrectomy with lymph node dissection (mostly D2) (14). H pylori was evaluated based on Giemsa stain of endoscopic biopsies or resected gastric tissue. Survival time was measured from date of tissue diagnosis to date of most recent follow up visit or date of death.
Recurrence-free survival (RFS) and overall survival (OS) were calculated from the date of diagnosis to the date of first radiographically documented recurrence, and date of last follow-up or death, respectively. The baseline characteristics and clinical and pathological outcomes were compared between two groups using chi-square, Fisher’s exact or Mann-Whitney U test. Multiple comparisons were carried out with Bonferroni corrections. Kaplan-Meier method and log-rank tests were used for survival analyses. All tests were two-sided with alpha level of 0.05. All analyses were performed in SAS v9.4 statistical software (SAS Institute, Cary, NC, USA)
Results
Ninety-five patients were identified. Demographic, disease characteristics, pathology, treatment and outcomes are summarized in Table 1. The majority of patients were male (n=58, 61%), white (36%) or Asian (34%), with median age at diagnosis 63–68. Tumors were stage I (51%), stage II (23%), stage III (25%), and stage IV (1%). H pylori infection status was documented at the time of cancer diagnosis in 89 (94%) patients, and following cancer diagnosis and treatment in 6 (6%) patients. All 6 of these patients were H pylori negative and had no documented history of prior treatment for H pylori.
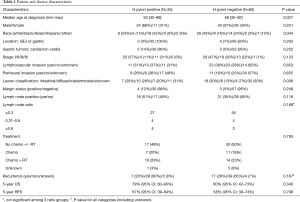
Full table
Younger age at diagnosis and Asian race were associated with H pylori positive GC based on univariate analysis. There was a higher proportion of Caucasians in the H pylori negative group (45% vs. 24%). There were a total of four GEJ cancers, which were all H pylori negative.
Lauren histologic classification was associated with H pylori infection, though pair-wise comparisons of the different subtypes (intestinal, diffuse, indeterminate, unknown) were not significantly different. There was no difference in cancer therapies (chemotherapy/radiation) according to H pylori status. None of these cancers were considered Barrett related since there was no documented Barrett metaplasia in pathology reports.
There was a trend towards better outcomes in H pylori positive patients. H pylori positive patients exhibited increased 5-year OS [79% (95% CI: 60–90%) vs. 60% (95% CI: 42–73%), P=NS] and 5-year RFS [67% (95% CI: 39–84%) vs. 58% (95% CI: 39–74%), P=NS] compared to H pylori negative patients. Outcomes remained improved in H pylori positive patients after controlling for prognostic factors including stage, treatment, lymphovascular invasion and perineural invasion [OS HR =1.56 (95% CI: 0.57–4.23), RFS HR =1.57 (95% CI: 0.53–4.63), P=NS]. The trend persisted after the exclusion of patients with a history of prior malignancy (N=10, one each of papillary thyroid, hepatocellular carcinoma, lung, follicular lymphoma, renal cell carcinoma, colon, uterine, prostate, and two breast cancers) [OS HR =2.73 (95% CI: 0.81–9.06), RFS HR =2.47 (95% CI: 0.70–8.71), P=NS] and patients whose H pylori status was not documented at time of GC or GEJ cancer diagnosis [OS HR =1.31 (95% CI: 0.46–3.78), RFS HR =1.21 (95% CI: 0.31–4.71), P=NS].
Discussion
The importance of H pylori status on outcomes in GC after curative resection has been studied with mixed results. Multiple studies have not been able to show a meaningful relationship between H pylori infection and prognosis. In a group of 157 patients with GC who underwent curative surgery in China, H pylori positivity and quantitative PCR were related to N staging, but were not associated with a difference in OS or RFS (15). These findings have been replicated in similar populations (16), as well as in distal gastric cancer (17), stage IV noncardiac gastric cancer (18) and proximal gastric cancer crossing the GEJ (19). There are however some studies that report negative outcomes with H pylori infection. Li and colleagues showed that H pylori positive status was associated with poorer disease specific survival (DSS) and RFS and that H pylori infection could be an independent predictor of prognosis in GC (20).
There is some data to support a protective benefit of H pylori infection. A German study of 166 GC patients undergoing curative resection between 1992–2002, showed higher RFS (56.7 vs. 19.2 months, HR =2.16; P<0.05) and OS (61.9 vs. 19.2 months, HR =2; P<0.05) in H pylori infected cancers compared to uninfected patients (21). Similarly, an Italian study showed that H pylori positivity independently predicted long term survival [HR =2.47, (1.40–4.35), P=0.002] (22). Another study of 559 patients showed increased OS (84.3 vs. 44.2 months, P=0.008) for H pylori positive compared to negative patients, though there was no difference in RFS or disease specific survival (23).
In the current series, H pylori positive patients tended to have better survival outcomes compared to H pylori negative patients but the differences were not significant, likely owing to the small sample size. Nevertheless, the trend remained consistent after adjusting for poor prognostic factors such as lymphatic and/or blood vessel invasion (24-26), perineural invasion (27), stage and treatment.
Several hypotheses might explain the improved outcomes seen in H pylori positive versus negative patients. There is some evidence that tumor specific immune responses may be enhanced in H pylori positive patients. In one study, H pylori positive gastric tumors had fewer regulatory T cells than negative tumors (21). Autoantibodies generated against H pylori components may also demonstrate cross-reactivity against GC cells which express H pylori antigens or mimic molecules (28). Furthermore, a higher frequency of microsatellite instability (MSI) has been observed in H pylori positive than negative GCs (29,30) which may also confer a more favorable prognosis, similar to what is observed in colorectal cancer. Given recent data showing MSI to be a strong predictor of response to immune checkpoint inhibitors (31), it would be interesting to explore whether responses to these agents differ by H pylori status.
We observed that H pylori infected patients were younger and were more frequently Asian compared to H pylori uninfected patients. The low frequency of GEJ cancers encountered is consistent with the literature that H pylori is primarily a risk factor for GC and less for GEJ cancer. H pylori infection can cause atrophic gastritis and induce specific cytokines that result in decreased acid production. Reduction in acid production and resultant decreased risk of gastroesophageal reflux may explain why GEJ cancer is less commonly associated with H pylori (32). Our results showed that H pylori positive tumors were more frequently diffuse/mixed histology rather than intestinal type. Although H pylori is more commonly associated with intestinal type, it can be seen with diffuse type too (33,34). The lymph node (LN) ratio and number of metastatic LN have been shown to impact survival in GC (25) To our knowledge, there are no studies that comment on the extent of lymph node involvement based on H pylori status. Our results show no difference in the lymph node ratio according to H pylori status.
This study is limited by its retrospective design and small sample size providing insufficient power to discern differences between the groups. Only a limited number of patients had documented H pylori status, and we were unable to determine if H pylori negative patients had previously been positive and treated. There is also some potential for case misclassification since H pylori status was discerned by histopathology alone, and patient use of anti secretory therapy was unknown.
In conclusion, this retrospective series adds to the body of literature reporting a trend towards improved GC outcomes among H pylori infected compared to uninfected patients. Further studies are needed to explore the molecular underpinnings and possible therapeutic implications of these differences.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of the Mount Sinai Hospital (No. FWA00005656).
References
- GLOBOCAN 2012: Estimated Cancer Incidence and Prevalence Worldwide in 2012. IARC/WHO [Internet]. [cited 2015 Mar 23]. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx#
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311-5. [Crossref] [PubMed]
- Ma S, Ma Q, Li J, et al. Meta-analysis on relationship between Helicobacter pylori infection and esophagogastric junction adenocarcinoma. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:418-24. [PubMed]
- Eslick GD, Lim LL, Byles JE, et al. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol 1999;94:2373-9. [Crossref] [PubMed]
- Eurogast Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet 1993;341:1359-62. [Crossref] [PubMed]
- Ahn HJ, Lee DS. Helicobacter pylori in gastric carcinogenesis. World J Gastrointest Oncol 2015;7:455-65. [PubMed]
- Kuipers EJ. Review article: exploring the link between Helicobacter pylori and gastric cancer. Aliment Pharmacol Ther 1999;13 Suppl 1:3-11. [Crossref] [PubMed]
- Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter 2014;19 Suppl 1:1-5. [Crossref] [PubMed]
- Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol 2010;25:479-86. [Crossref] [PubMed]
- Lee YC, Chiang TH, Chou CK, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016;150:1113-1124.e5. [Crossref] [PubMed]
- Ford AC, Forman D, Hunt R, et al. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane database Syst Rev 2015;7:CD005583. [PubMed]
- Shichijo S, Hirata Y, Niikura R, et al. Histological intestinal metasplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc. American Society for Gastrointestinal Endoscopy 2016;1-7.
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Qiu HB, Zhang LY, Keshari RP, et al. Relationship between H.Pylori infection and clinicopathological features and prognosis of gastric cancer. BMC Cancer 2010;10:374. [Crossref] [PubMed]
- Posteraro B, Persiani R, Dall’Armi V, et al. Prognostic factors and outcomes in Italian patients undergoing curative gastric cancer surgery. Eur J Surg Oncol 2014;40:345-51. [Crossref] [PubMed]
- Santos RS, Lourenço JE V, Augusto F, et al. Artigo Original / Original Article Helicobacter Pylori Has No Influence On 2011;(2):109-11.
- Syrios J, Sougioultzis S, Xynos ID, et al. Survival in patients with stage IV noncardia gastric cancer - the influence of DNA ploidy and Helicobacter pylori infection. BMC Cancer 2012;12:264. [Crossref] [PubMed]
- Zhang YF, Shi J, Yu HP, et al. Factors predicting survival in patients with proximal gastric carcinoma involving the esophagus. World J Gastroenterol 2012;18:3602-9. [Crossref] [PubMed]
- Li G, Wang Z, Wang Z, et al. HP Gastric cancer patients with Helicobacter pylori infection have a poor prognosis. J Surg Oncol 2013;108:421-6. [Crossref] [PubMed]
- Meimarakis G, Winter H, Assmann I, et al. Helicobacter pylori as a prognostic indicator after curative resection of gastric carcinoma: a prospective study. Lancet Oncol 2006;7:211-22. [Crossref] [PubMed]
- Marrelli D, Pedrazzani C, Berardi A, et al. Negative Helicobacter pylori status is associated with poor prognosis in patients with gastric cancer. Cancer 2009;115:2071-80. [Crossref] [PubMed]
- Postlewait LM, Squires MH 3rd, Kooby DA, et al. Preoperative Helicobacter pylori Infection is Associated with Increased Survival After Resection of Gastric Adenocarcinoma. Ann Surg Oncol 2016;23:1225-33. [Crossref] [PubMed]
- del Casar JM, Corte MD, Alvarez A, et al. Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol 2008;134:153-61. [Crossref] [PubMed]
- Zhang M, Wang J, Shi W, et al. Prognostic significance of metastatic lymph nodes ratio in patients with gastric adenocarcinoma after curative gastrectomy. Chin Med J (Engl) 2014;127:1874-8. [PubMed]
- Lee JH, Kim MG, Jung MS, et al. Prognostic significance of lymphovascular invasion in node-negative gastric cancer. World J Surg 2015;39:732-9. [Crossref] [PubMed]
- Selçukbiricik F, Tural D, Büyükünal E, et al. Perineural invasion independent prognostic factors in patients with gastric cancer undergoing curative resection. Asian Pac J Cancer Prev 2012;13:3149-52. [Crossref] [PubMed]
- Xue LJ, Su QS, Yang JH, et al. Autoimmune responses induced by Helicobacter pylori improve the prognosis of gastric carcinoma. Med Hypotheses 2008;70:273-6. [Crossref] [PubMed]
- Kim JJ, Tao H, Carloni E, et al. Helicobacter pylori impairs DNA mismatch repair in gastric epithelial cells. Gastroenterology 2002;123:542-53. [Crossref] [PubMed]
- Li JH, Shi XZ, Lv S, et al. Effect of Helicobacter pylori infection on p53 expression of gastric mucosa and adenocarcinoma with microsatellite instability. World J Gastroenterol 2005;11:4363-6. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Xu W, Liu Z, Bao Q, et al. Viruses, Other Pathogenic Microorganisms and Esophageal Cancer. Gastrointest tumors 2015;2:2-13.
- Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis 2012;13:2-9. [Crossref] [PubMed]
- Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001;49:347-53. [Crossref] [PubMed]


