|
Case Report
Sustained response to FOLFOX and Bevacizumab in metastatic bronchial carcinoid – A case report and review of the literature
Ikechukwu Akunyili1, Monica T Garcia-Buitrago2, Jessica MacIntyre1, Joe Levi3, Caio M Rocha Lima1
1Department of Hematology and Oncology, University of Miami, Miami, FL; 2Department of Pathology, University of Miami, Miami, FL; 3Department of Surgical Oncology, University of Miami, Miami, FL
Corresponding to: Ikechukwu Akunyili, MD. Hematology and Oncology, University of Miami, 1475 NW 12 Avenue Miami 33136 Florida, USA. Tel: 3052438644; Fax: 3052438644. E-mail: iakunyili@med.miami.edu.
|
|
Abstract
A 65 year old male with bronchial carcinoid developed diffuse liver metastases seventeen months after curative resection
of a solitary liver metastasis. Treatment with modified (m) FOLFOX 6 (5-FU, 400mg/m2 bolus infusion, followed by
Leucovorin 400 mg/m2 and Oxaliplatin 85 mg/m2 given in “Y” over 2 hours followed by 5 FU 2,400 mg/m2 continuous
infusion over 46 hours) plus Bevacizumab and Octreotide were given after progression on Octreotide alone. It resulted
in sustained partial response. To our knowledge, this is the first reported case demonstrating activity of FOLFOX and
Bevacizumab in metastatic bronchial carcinoid. The response and clinical benefit of FOLFOX with Bevacizumab in this
case of metastatic bronchial carcinoid suggest that this treatment is active and should be further studied in patients with
metastatic and unresectable bronchial carcinoid tumors.
Key words
FOLFOX, Bevacizumab, Bronchial carcinoid
J Gastrointest Oncol 2011; 2: 117-121. DOI: 10.3978/j.issn.2078-6891.2011.016
|
|
Introduction
Bronchial carcinoids are neuroendocrine tumors of foregut
origin typically considered benign. The WHO classifies
the tumors as typical or atypical carcinoids. Bronchial
carcinoids rarely metastasize to the liver. Metastatic
carcinoids can be cured in some instances by hepatic
resection however there is a significant rate of relapse
following curative resection of neuroendocrine tumors
at five years ( 1, 2). In patients with midgut metastatic
neuroendocrine carcinoma octreotide may provide an
improvement in progression free survival (PFS) over
placebo ( 3). The role of chemotherapy and antiangiogenic
therapy in this disease is evolving. We present a patient
with diffuse hepatic metastatic from bronchial carcinoid, who had a durable partial response to octreotide plus
mFOLFOX6 and bevacizumab.
|
|
Case presentation
A 65-year-old male nonsmoker who in November 2000
underwent a r ight middle lobectomy with negative
margins followed by adjuvant chemoradiation therapy with
carboplatin, paclitaxel as radiation sensitizers at an outside
facility for the diagnosis of atypical bronchial carcinoid.
Post-surgery, the patient was disease free for over five years.
In October 2006, surveillance Octreotide scans
demonstrated increased activity in the left hepatic lobe, with
non-specific activity in the right lung. CT scan revealed a
hypodense mass in the left hepatic lobe and a biopsy of this
mass was obtained. The biopsy was positive for metastatic
neuroendocrine tumor and immunohistochemical studies
were positive for chromogranin. The patient was treated
with a combination of cisplatin and etoposide starting in
December 2006, but developed progressive disease in the
liver after four months. He was then switched to carboplatin
and paclitaxel in April 2007 and following three cycles of
this regimen he was evaluated in our institution for a second
opinion.
On initial assessment, he had no chest tightness,
productive cough, shortness of breath or palpitations. He
also had no diarrhea, abdominal pain, nausea, vomiting
or f lushing. His past medical history was positive for
hypertension controlled on Amlodipine 10mg daily.
Physical examination: Eastern Cooperative Oncology
Group (ECOG) performance status of one. He had an
enlarged and palpable left hepatic lobe, with normal
cardiopulmonary examination.
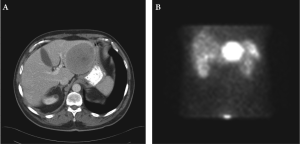
A computed tomography (CT) scan of the chest,
abdomen and pelvis demonstrated a well-circumscribed
mass occupying the bulk of the left hepatic lobe (7.7 x
9.0 cm) (Figure 1A and 1B). A biopsy specimen of the
mass was consistent with metastatic neuroendocrine
carcinoma. The chromogranin A level was 468 ng/mL
(normal ≤ 36.4 ng/mL). The patient’s case was reviewed in
our multidisciplinary tumor board and he was considered
potentially resectable. Subsequently, in August 2007, he
underwent surgical resection of segments II and III of the
liver and intraoperative examination revealed that the
tumor was located in these two segments.
Pathologic examination of the specimen demonstrated
an encapsulated, 8 x 8 x 6.5 cm well-differentiated
neuroendocrine tumor morphologically consistent with
metastatic carcinoid to liver. The resection margins were
negative and lymphovascular invasion was identified.
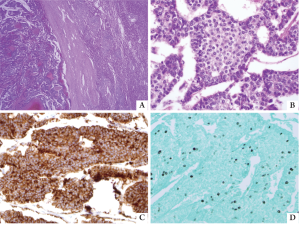
Immunohistochemical staining was positive for
chromogranin and synaptophysin and negative for insulin,
glucagon, serotonin, calcitonin, bombexin, TTF and
CDX-2 (Figure 2). The Ki-67 immunostain showed a low
proliferative activity. The patient had an uneventful postoperative
course. Approximately 1 month after resection,
his serum chromogranin A level was 19 ng/mL (normal ≤
15 ng/mL) and repeat octreotide scan again demonstrated
non-specific increased activity in the right lower lung.
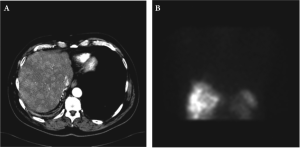
Seventeen months after the surgical resection, octreotide scan demonstrated increased activity in the right lobe of
the liver, skull, humerus and ribs in addition to persistent
uptake in the right lower lung. The CT scan demonstrated
innumerable hypodense lesions in both hepatic lobes.
Therapy was commenced with the long acting somatostatin
analogue (Octreotide LAR) monthly with initial stable
disease. After nine months of therapy with Octreotide
LAR, he developed progressive disease, with rise in the
serum chromogranin from 340 to 2980 (normal ≤ 36.4
ng/mL) and increased uptake of octreotide in the bones on
Octreoscan in addition to progressive disease in the liver.
(Figure 3A and 3B).
He was started on modified FOLFOX 6 (5-FU, 400 mg/
m2 bolus infusion, followed by Leucovorin 400 mg/m2 and
Oxaliplatin 85 mg/m2 given in “Y” over 2 hours followed
by 5 FU 2,400 mg/m2 continuous infusion over 46 hours)
plus bevacizumab in addition to Octreotide LAR and
zolendronic acid in October 2009, with achievement
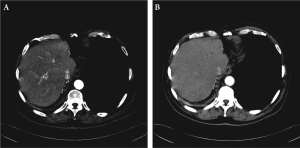
of partial response by RECIST criteria, as noted on the
CT scan obtained after 9 and 18 cycles of chemotherapy
(Fig 4A and 4B respectively) The serum chromogranin
A level decreased to 424 by December 2010. He received
26 cycles of mFOLFOX6 plus bevacizumab administered
every 2 weeks over 16 months without dose reduction.
The treatment was well tolerated, and he experienced
NCI-CTC grade 2 tinnitus with sensorineural hearing
impairment that did not require treatment; grade 2
mucositis, anemia and neutropenia. He also had grade 1
thrombocytopenia, proteinuria and peripheral sensory
neuropathy. He had no grade 3 or 4 adverse events. Upon
the development of grade 2 tinnitus, the treatment with
modified FOLFOX 6 was delayed for two weeks to enable
full audiology and otolaryngology evaluation. On occasion
the patient had treatment delays for personal reasons. In
February 2011 restaging imaging studies demonstrated
progressive disease in the liver and bones and he was switched to everolimus.
|
|
Discussion
Bronchial carcinoid tumors are neuroendocrine neoplasms
of foregut origin which are generally considered low grade
neoplasms. These tumors usually present with respiratory
symptoms such as cough, wheezing, hemoptysis, and
recurrent pneumonias ( 3-5). Carcinoid tumors greater
than 5mm in diameter are classified as typical or atypical
based on the mitotic activity and necrosis. Typical features
include mitotic activity in fewer than 2 cells per 10 HPF and
absence of focal necrosis. Atypical features include greater
mitotic activity and punctuate necrosis ( 3, 5, 6). Metastasis
to regional lymph nodes occurs in less than 15% of typical
bronchial carcinoids, but may be present in 30% to 50%
of atypical tumors ( 4, 5). Certain features, like extension
along the bronchial tree, may increase the risk of metastasis
of typical bronchial carcinoids ( 7) Peripheral tumors with
typical features are preferably removed with a large wedge
or segmental resection, whereas more radical procedures,
such as lobectomy with lymph node sampling, bi-lobectomy,
sleeve resection, or pneumonectomy, are often chosen for
central or atypical carcinoids. The long-term postoperative
survival is 83% to 96% for typical carcinoids and 31% to
79% for atypical carcinoids ( 4-6). Resection of metastasis may have a curative role in
neuroendocrine cancers, however, about 90% of patients
with liver metastases have bilateral and multifocal hepatic
metastases and only 10-25% of patients have tumors that
are sufficiently localized to allow for a curative resection
( 1, 8). In selected patients with resectable liver metastases,
surgery provides both a symptomatic relief and a potential
survival benefit (5-year actuarial survival of 18% to 29%
without surgery, increasing to 50% to 79% after resection)
( 8, 9). Despite the multifocal and unresectable nature of
many patients with liver metastases, the clinical course can
be prolonged and debilitating with pain due to progressive
increase in liver size and development of carcinoid
syndrome in patients with hormonally active cancers ( 8).
Debulking surgery with resection of greater than 90% of
gross tumor in patients whose tumors cannot be completely
excised provided both a palliative and a potential survival
benefit ( 1, 10). Metastatic carcinoid tumors are relatively chemoresistant
( 4, 11, 12). However, oxaliplatin in combination with a
fluoropyrimidine has demonstrated activity in metastatic
neuroendocrine tumors ( 11, 13, 14). However, we are
unaware of any reported case of a patient with metastatic
bronchial carcinoid treated with FOLFOX or XELOX
(capecitabine and oxaliplatin in combination). In patients with well differentiated neuroendocrine tumors of the
gastro-entero-pancreatic region, the combination of
capecitabine and oxaliplatin had a clinical benefit of 78%
(30%PR and 48%SD) ( 15). Somatostatin analogues have
been historically used in patients with NET for symptom
palliation. However, antitumor effect was not demonstrated
until recently. The PROMID study group demonstrated
that Octreotide LAR significantly improved the PFS from
6.6 to 14.3 months over placebo in patients with functional
and non-functional midgut NETs ( 16). The hypervascular nature of neuroendocrine carcinomas
makes them an interesting target for antiangiogenesis
agents. In patients with well differentiated pancreatic
neuroendocrine tumor, a recent phase 3 clinical trial with
the antiangiogenesis agent sunitinib showed a significant
improvement in PFS over placebo, from 5.5 to 11.3 months
( 17). In an earlier phase 2 trial, sunitinib demonstrated
a clinical benefit of 85.4% (2.4% ORR and 83% SD) in
patients with advanced carcinoid; however, the authors did
not specify how many patients had stable disease at study
entry and the ORR in carcinoids was less than the 16.7%
observed in pancreatic NET ( 18). Bevacizumab with and
without IFN has shown activity in neuroendocrine tumors
( 19, 20). Preliminary data of a small Phase II clinical trial
of FOLFOX and bevacizumab administered every 2 weeks
in patients with advanced and progressive NETs including
carcinoid tumors demonstrated promising clinical activity,
with 20% PR and 80% SD in the patients with carcinoid
( 21). The patients received a median of 11 cycles (range 3
to 26) of chemotherapy with 30% Grade 3-4 neutropenia,
38% grade 3-4 fatigue and 23% grade 3-4 hypertension ( 21).
Preliminary results presented at the 2010 ASCO annual
meeting from another phase II clinical trial of XELOX plus
bevacizumab in 31 patients with predominantly metastatic
unresectable enteropancreatic NETs showed a clinical
benefit ratio of 94% (23% PR and 71% SD). However, it is
unclear if any of the patients enrolled in these studies of
XELOX or FOLFOX with bevacizumab had metastatic
bronchia l ca rcinoid ( 20-22). The MTOR inhibitor
everolimus has demonstrated activity in NETs; in phase
II clinical trial involving patients with low to intermediate
grade NET, everolimus achieved a PR of 17% and 27 % in
carcinoid tumors and pancreatic NET respectively ( 23).
A recent phase III clinical trial of everolimus compared to
placebo in patients with progressive metastatic pancreatic
NET demonstrated a statistically significant increase in PFS
from 4.6 months to 11months in favor of everolimus ( 24).
The result of the recent phase III clinical trial RADIANT-2
in patients with non-pancreatic NETs including bronchial
carcinoids, showed that the combination of everolimus and
octreotide led to a 5.1 month increase in PFS compared to octreotide plus placebo (16.4 vs. 11.3 months); however,
this did not meet the predetermined statistical end point
( 25). This is the first case of a patient with bronchial carcinoid
treated with FOLFOX and bevacizumab. FOLFOX
and XELOX with or without bevacizumab appear to be
a very attractive chemotherapy regimen in metastatic
neuroendocrine tumors. The response and clinical benefit
of FOLFOX with bevacizumab in this case suggest that this
treatment is active and should be further studied in patients
with metastatic and unresectable bronchial carcinoid
tumors. The emergence of new treatment options in NET is
exciting; however the place of these agents in the treatment
algorithm of NET remains to be better defined.
|
|
References
- Mazza fer ro V, Pulv irenti A, Coppa J. Neuroendocr ine tumors
metastatic to the liver: how to select patients for liver transplantation? J
Hepatol 2007;47:460-6.[LinkOut]
- Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que
FG. Surgical treatment of neuroendocrine metastases to the liver: a plea
for resection to increase survival. J Am Coll Surg 2003;197:29-37.[LinkOut]
- Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med
1999;340:858-68.[LinkOut]
- Kulke MH. Clinical presentation and management of carcinoid tumors.
Hematol Oncol Clin North Am 2007;21:433-55.[LinkOut]
- Phillips JD, Yeldandi A, Blum M, de Hoyos A. Bronchial carcinoid
secreting insulin-like growth factor-1 with acromegalic features. Ann
Thorac Surg 2009;88:1350-2.[LinkOut]
- Naalsund A, Rostad H, Strøm EH, Lund MB, Strand TE. Carcinoid
lung tumors - incidence, treatment and outcomes: a population-based
study. Eur J Cardiothorac Surg 2011;39:565-9.[LinkOut]
- Das-Neves-Pereira JC, de Matos LL, Danel C, Trufelli D, Riquet
M. Typica l bronchopulmonary carcinoid tumors: a ramifying
bronchial presentation with metastatic behavior. Ann Thorac Surg
2006;82:2265-6.[LinkOut]
- Ihse I, Persson B, Tibblin S. Neuroendocrine metastases of the liver.
World J Surg 1995;19:76-82.[LinkOut]
- Grazi GL, Cescon M, Pierangeli F, Ercolani G, Gardini A, Cavallari A,
et al. Highly aggressive policy of hepatic resections for neuroendocrine
liver metastases. Hepatogastroenterology 2000;47:481-6.[LinkOut]
- Que FG, Nagorney DM, Batts KP, Linz LJ, Kvols LK. Hepatic resection
for metastatic neuroendocrine carcinomas. Am J Surg 1995;169:36-43.[LinkOut]
- Tetzlaff ED, Ajani JA. Oxaliplatin-based chemotherapy for the
treatment of a metastatic carcinoid tumor. Int J Gastrointest Cancer
2005;36:55-8.[LinkOut]
- Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical
review. Oncologist 2005;10:123-31.[LinkOut]
- Pape U, Tiling N, Bartel C, Plöckinger C, Wiedenmann B, et al.
Oxaliplatin plus 5-f luorouracil/folinic acid as palliative treatment for progressive malignant gastrointestinal neuroendocrine carcinomas. J
Clin Oncol 2006;24:s14074.
- Garin L, Corbinais S, Boucher E, Blanchot J, Le Guilcher P, Raoul JL.
Adenocarcinoid of the appendix vermiformis: complete and persistent
remission after chemotherapy (folfox) of a metastatic case. Dig Dis Sci
2002;47:2760-2.[LinkOut]
- Bajetta E, Catena L, Procopio G, De Dosso S, Bichisao E, Ferrari L,
et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments
for progressing low-grade and high-grade neuroendocrine tumours?
Cancer Chemother Pharmacol 2007;59:637-42.[LinkOut]
- Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied
M, et al. Placebo-controlled, double-blind, prospective, randomized
study on the effect of octreotide LAR in the control of tumor growth in
patients with metastatic neuroendocrine midgut tumors: a report from
the PROMID Study Group. J Clin Oncol 2009;27:4656-63.[LinkOut]
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C,
et al. Sunitinib malate for the treatment of pancreatic neuroendocrine
tumors. N Engl J Med 2011;364:501-13.[LinkOut]
- Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, et al.
Activity of sunitinib in patients with advanced neuroendocrine tumors.
J Clin Oncol 2008;26:3403-10.[LinkOut]
- Yao JC, Phan A, Hoff PM, Chen HX, Charnsangavej C, Yeung SC, et
al. Targeting vascular endothelial growth factor in advanced carcinoid
tumor: a random assignment phase II study of depot octreotide
with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol
2008;26:1316-23.[LinkOut]
- Fazio N, Cinieri S, Lorizzo K, Squadroni M, Orlando L, Spada F, et al.
Biological targeted therapies in patients with advanced enteropancreatic
neuroendocrine carcinomas. Cancer Treat Rev 2010;36:S87-94.[LinkOut]
- Venook AP, Ko AH, Tempero MA, Uy J, Weber T, Korn M, et al.
Phase II trial of FOLFOX plus bevacizumab in advanced, progressive
neuroendocrine tumors[abstract]. J Clin Oncol 2008;26:s15545.
- Kunz PL, Kuo T, Zahn JM, Kaiser HL, Norton JA, Visser BC, et al.
A phase II study of capecitabine, oxaliplatin, and bevacizumab for
metastatic or unresectable neuroendocrine tumors. J Clin Oncol
2010;28:s4104.
- Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, et al. Efficacy
of RAD001 (everolimus) and octreotide LAR in advanced low- to
intermediate-grade neuroendocrine tumors: results of a phase II study.
J Clin Oncol 2008;26:4311-8.[LinkOut]
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al.
Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J
Med 2011;364:514-23.[LinkOut]
- Yao JC, Hainsworth JD, Baudin E, Peeters M, Hoersch D, Anthony
LB, et al. Everolimus plus octreotide LAR (E+O) versus placebo plus
octreotide LAR (P+O) in patients with advanced neuroendocrine
tumors (NET): Updated results of a randomized, double-blind,
placebo-controlled, multicenter phase III trial (RADIANT-2)[abstract].
J Clin Oncol 2011;29:s159.
Cite this article as:
Akunyili I, Garcia-Buitrago M, MacIntyre J, Levi J, Lima C. Sustained response to FOLFOX and Bevacizumab in metastatic bronchial carcinoid – A case report and review of the literature. J Gastrointest Oncol. 2011;2(2):117-121. DOI:10.3978/j.issn.2078-6891.2011.016
|