Concurrent chemoradiation with volumetric modulated Arc therapy of patients treated for anal cancer—acute toxicity and treatment outcome
Introduction
Concurrent chemoradiation is currently a mainstay for patients with anal cancer after the publication of Nigro trial (1). Clinical results are excellent with high rates of disease-free survival (DFS), overall survival (OS) and locoregional control (LC). Combinations of fluorouracil-based and mitomycin C (MMC) chemoradiation have been established as the standard of care, leading to a complete tumor regression in 80–90% of patients, with locoregional failures (LRF) in nearly 15% (2).
Radiotherapy techniques have evolved from simple two-dimensional (2D) and three-dimensional (3D) ones to conformal 3D (3D CRT) and ultimately to intensity modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT) or helical chemotherapy (HT).
Early randomized trials (3,4) confirmed excellent clinical results in case of radiotherapy and chemotherapy combination, but significant acute dermatologic, gastrointestinal, genitourinary and hematologic toxicity. ACT II trial (5) showed that the combination of fluorouracil and MMC with radiotherapy remains the standard treatment without maintenance chemotherapy. Long-term update of RTOG 98-11 trial (6) excluded platinum based chemotherapy as induction treatment and confirmed high rates of acute non-hematologic toxicity (74%).
Concurrent chemoradiation induces significant acute and late toxicity (3-6) and consequently, in older trials; a 2 weeks gap was set to allow recovery. Thanks to new advanced high precision radiotherapy techniques as IMRT and more recently VMAT it is possible to reduce doses delivered to critical organs at risk; and therefore the acute toxicities; during and after the treatment. That is the case mainly for the skin and the gastrointestinal tract (7).
Here we present our clinical results and data on acute dermatologic, gastrointestinal and genitourinary toxicity of an homogeneous group of patients diagnosed with anal cancer and treated with VMAT plus concurrent chemotherapy between February 2011 and April 2016.
Methods
Eligibility criteria and staging:
All patients had a histologically confirmed diagnosis of squamous cell carcinoma of the anal canal.
Our institution standard staging workup included digital rectal examination, endoscopic ultrasound, thorax-abdomino-pelvic computed tomography (CT) and pelvic magnetic resonance imaging (MRI). In case of doubtful reports we staged the patients with PET/CT. Patients with distant metastasis were excluded from this analysis. HIV positive patients were treated as the HIV negative ones. Staging was performed according to AJCC staging manual 7th edition (8).
Radiotherapy planning and delivery
CT virtual simulation in “frog legged” supine position with knee support and comfortably full bladder was performed for all patients. CT scan with 3 mm slice thickness was performed from L2 to mid femoral bones. The isocentre was identified on the CT and marked on patient skin with 3 permanent tattoos.
Gross tumor volume (GTV) encompassed the primary tumor and all the involved lymph nodes according to CT, MRI and PET images.
Clinical target volume (CTV) covered GTV with 2 cm margin, isotropically, with subsequent optimization for avoiding bones and critical structures. Elective lymph nodes were the external and internal iliac, presacral, perirectal and bilateral inguinal ones. Nodal areas were delineated with 7 mm margin around the blood vessels; optimization for excluding bones was applied for these volumes, too.
Planning target volume (PTV II) was generated adding 7 mm isotropically to the CTV and then modified according to the radiation oncologist decision.
The CTV boost volume was the GTV (tumor and lymph nodes) plus 2 cm margin, the PTV I (boost) was obtained adding 7 mm to the CTV.
The small and large bowel (contoured as cavity), the bladder and the femoral heads were contoured as organs at risk.
Clinical planning objectives were: V40Gy <50% for the bladder, V30Gy <450 cm3 for the bowel (defined as the entire bowel “bag”), V40Gy <25% and D1% <50 Gy for the femoral heads (Vx Gy is the volume of a structure receiving at least x Gy while Dx% is the dose received by at maximum x% of an organ).
Dose prescription was 39.6 Gy, 1.8 Gy/fraction for the PTV II, the PTV I doses were 14.4 Gy up to a total dose of 54 Gy in 4 patients and 19.8 up to 59.4 Gy in 15 patients. One patient received a boost dose of 18 Gy up to a total dose of 57.6 Gy and another one was treated with 36 Gy and boost of 19.8 Gy up to a total dose of 55.8 Gy.
All treatment plans were developed using the Varian Eclipse planning system (version 12, Varian Medical System, Palo Alto, CA, USA) and patients were treated with VMAT Rapid Arc technique (Varian Medical System, Palo Alto, CA, USA) with two arcs of 6 MV photon beams generated by either a Varian Clinac 2100 or a True Beam linear accelerator .
Chemotherapy
Seventeen patients received two cycles of MMC 10 mg/m2 on day 1 and day 29 as bolus IV injection and 5-FU 1,000 mg/m2 as continuous infusion, four patients received one cycle of MMC 12 mg/m2 on day 1 and Capecitabine 825 mg/m bid, 5 days per week.
Acute toxicity
We considered as acute toxic effects all the events occurred within 3 months after the end of chemoradiation. They were evaluated and registered according to Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0.
We evaluated gastrointestinal, genitourinary and dermatologic toxicity weekly during the radiotherapy and then monthly until 90 days after the treatment completion.
Follow up and clinical results
All patients were checked weekly during the treatment by a trained radiation oncologist, and then medical examinations were scheduled after 1 month and subsequently every 3 months during the first year and every 6 months thereafter. Digital rectal examination and anoscopy were performed during every follow-up visit. MRI was performed at 24 weeks and anal canal biopsy was carried out in case of suspicious findings or partial clinical response. A salvage abdominoperineal resection (APR) was recommended in case of residual tumor, histologically confirmed by biopsy. Complete clinical response was confirmed if both clinical examination with anoscopy and MRI didn’t detect-signs of residual tumor.
Statistical analysis
All continuous data were expressed in terms of median and range. Each end point was calculated from the date of initial histological diagnosis using Kaplan-Meier method. Local control (LC) was defined as the absence of tumor in the treated area (in-field). Distance control was defined as the absence of tumor outside the treated area. DFS was defined as the time from diagnosis to the date of local and/or distant recurrence or death due to all causes whichever comes first. OS was defined as the time to death. Statistical analysis was carried out using SYSTAT version 11.0 (SPSS, Chicago, IL, USA).
Results
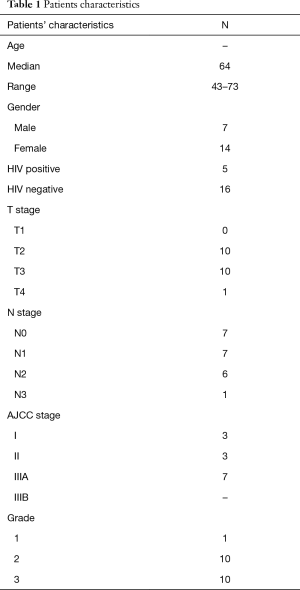
A total of 21 patients, 7 males and 14 females, treated in our institution between February 2011 and April 2016 were included in this analysis. Patient and tumor characteristics are summarized in Table 1.
Full table
The median age was 64 years (range, 43–73 years). Five patients (23.8%) were HIV positive and 16 were HPV negative. Only three patients (14.2%) were diagnosed with stage I disease, three with stage II (14.2%), seven with stage IIIA (33.3%) and eight with stage IIIB (38.1%).
Median total dose prescription was 58 Gy (range, 54–59.4 Gy) with 1.8Gy daily fraction. Mean overall treatment time was 57 days (range, 43–71 days).Fifteen patients (71.4%) had a planned treatment break longer than 5 days and six (28.6%) patients were treated without gap. See Table 2 for treatment details.
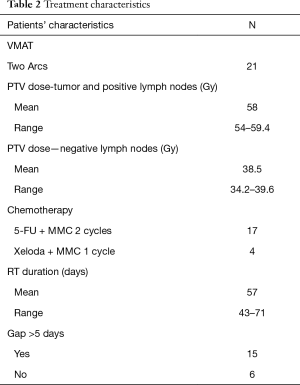
Full table
The median follow-up time was 35.5 months (range, 3–71 months). Three patients (14.2%) experienced local relapse and underwent salvage APR and one patient (4.7%) died due to distant metastases of unknown origin in lungs, liver and bones without local relapse.
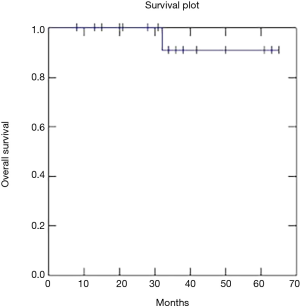
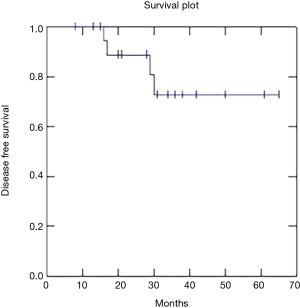
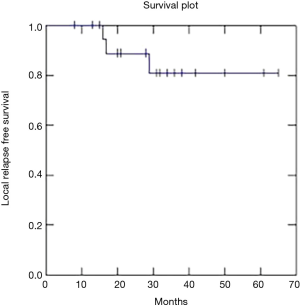
Two year OS, DFS and LC were respectively 91, 73 and 81% (Figures 1-3).
Treatment was very well tolerated with low acute toxicity rates. The details are reported in Table 3.The median duration of planned treatment break in fifteen patients was 11 days (range, 7−15 days) according to our institutional protocol at that time, independently on the acute side effects. The last six patients were treated without planned gap and they finished chemoradiation without breaks.

Full table
Acute dermatological toxicity, G3 (moist desquamation), was recorded in one patient, ten patients (47.6%) experienced a G2 skin toxicity while G1 toxicity was registered in eight patients (38%). Two patients completed the concomitant treatment without skin toxicity (G0).
One patient developed Grade 3 acute gastrointestinal toxicity, two patients (9.5%) experienced grade 2 acute GI toxicity (diarrhea with 4–6 stools per day) and ten patients (47.6%) G1 toxicity. Eight patients (42.8%) finished the combined treatment without acute gastrointestinal toxicity (G0).
Acute genitourinary toxicity G1 (microscopic hematuria; minimal increase in frequency, urgency, dysuria or nocturia; new onset of incontinence) was recorded in ten patients (47.6%) while eleven (52.4%) haven’t experienced genitourinary symptoms.
All patients except one completed concomitant chemotherapy. This patient was diagnosed with stroke during the treatment and the second cycle of 5-FU was withheld.
Discussion
Our data strongly support VMAT chemoradiation as treatment of choice for anal cancer patients. RT and concomitant chemotherapy is nowadays the standard of care for this patient, but acute/late toxicity and related treatment breaks are still an issue (2-6). Sophisticated techniques to deliver the prescribed dose to the target volumes can be used, in order to better spare the organs at risk at the same time. This results in reduced acute toxicity and allows avoiding treatment breaks.
A prospective randomized phase II trial, conducted by the radiotherapy oncology group (RTOG), confirmed the important role of IMRT in combination with chemotherapy for the reduction of acute morbidity in patients with anal canal cancer. This trial assessed the utility of dose-painted IMRT (DP-IMRT) in reducing the acute toxicity of 5-fluorouracil/MMC chemo-radiation for T2-4 N0-3 M0 anal cancer. Nevertheless the primary endpoint of reducing grade ≥2 combined gastrointestinal and genitourinary acute adverse events by 15%, comparing the results to the RTOG 9811 ones, was not met. However, DP-IMRT yielded significant sparing of acute grade 2≥ hematologic, and grade ≥3 dermatologic and gastrointestinal toxicity (9).
Direct comparison between 3D CRT and IMRT showed clear dosimetric benefits of the modern techniques over conformal radiation therapy. With IMRT we can spare organs at risk and reduce the acute toxicity rates and grades without compromising the PTV coverage. Dosimetric study of Menkarios (10) concluded that IMRT is superior to 3D CRT regarding the dose distribution in critical structures close to PTV.
Chuong et al. (11) compared IMRT and 3D CRT and reported that long-term outcomes did not significantly differ between RT techniques, but an important decrease of acute adverse effects and treatment breaks was achieved with IMRT.
VMAT, which combines rotational approach with continuous beam modulation, is a relatively new technique with excellent results in patients with anal cancer. Vieillot et al. (12) and Stieler et al. (13) compared directly VMAT and IMRT and showed equivalent coverage of PTV while VMAT plans allowed a better sparing of organs at risk, a significant reduction of monitor units (MU) and shorter treatment time .
Devisetty et al. (14) found that the volume of bowel receiving 30 Gy (V30) is strictly correlated with acute gastrointestinal (GI) toxicity in anal cancer patients treated with intensity-modulated radiation therapy plus concurrent chemotherapy; moreover they advised that it should be less than 450 cubic centimeters.
Han et al. (15) explored multiple dose-volume parameters and all were correlated moderately with acute gastrointestinal toxicity.
Our results are similar with median V30 equal to 438 cubic centimeters (range, 112–788 cc) resulting in low GI toxicity rates with only one case of G3 and without G4 grade toxicity. None of our patients had treatment break because of acute gastrointestinal adverse effects, even if fifteen patients had a planned gap according to our local guidelines at that time, independently on the symptoms.
The clinical outcomes in patients treated with conformal or IMRT techniques are similar as Dasgupta showed in her analysis of a large cohort of patients (16), confirming the new standard of radiation therapy delivery. Chuong et al. (17) reported 2 years LRC, OS and DFS of 94.6, 100 and 82.6% respectively in patients treated with IMRT based chemoradiation, registering minimal toxicity.
Recently Franco et al. (18) published excellent results with a 2-year DFS of 75.1% and OS of 85.2% using VMAT radiation therapy and chemotherapy. Our data confirm these results with 2-year DFS of 73%, OS of 91% and LC of 81%.
Our retrospective study included homogenous group of patients mainly with locally-advanced anal cancer (stage III: 71.4%). Excellent clinical results and low acute toxicity rates reproduce and confirm the published ones and confirm the role of chemoradiation with advanced techniques as VMAT as standard of care for curative treatment of anal cancer patients.
Conclusions
VMAT is an effective and safe radiotherapy technique, and today it should be considered as the standard treatment in combination with chemotherapy for patients with locally advanced anal cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: We confirm that Informed consent was taken from all the patients. The treatment modalities (VMAT and chemotherapy) were approved as standard of care in our institution, Oncology Institute of Southern Switzerland (No. Req-2017-00120), so the ethics board approval was automatic.
References
- Nigro ND. An evaluation of combined therapy for squamous cell cancer of the anal canal. Dis Colon Rectum 1984;27:763-6. [Crossref] [PubMed]
- Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol 2014;111:330-9. [Crossref] [PubMed]
- Epidermoid anal cancer: Results from the UKCCCR randomized trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin: UKCCCR Anal Cancer Trial Working Party—UK Co-ordinating Committee on Cancer Research. Lancet 1996;348:1049-54. [Crossref] [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Group. J Clin Oncol 1997;15:2040-9. [Crossref] [PubMed]
- James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, openlabel, 2 ×2 factorial trial. Lancet Oncol 2013;14:516-24. [Crossref] [PubMed]
- Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98–11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/ cisplatin. J Clin Oncol 2012;30:4344-51. [Crossref] [PubMed]
- Bazan JG, Hara W, Hsu A, et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011;117:3342-51. [Crossref] [PubMed]
- Anus. In: Edge SB, Byrd DR, Compton CC, et al. editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010:167-9.
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [Crossref] [PubMed]
- Menkarios C, Azria D, Laliberte B, et al. Optimal organ sparing intensity-modulated radiation therapy (IMRT) regimen for the treatment of locally advanced anal canal carcinoma: A comparison of conventional and IMRT plans. Radiat Oncol 2007;2:41. [Crossref] [PubMed]
- Chuong MD, Freilich JM, Hoffe SE, et al. Intensity-Modulated Radiation Therapy vs. 3D Conformal Radiation Therapy for Squamous Cell Carcinoma of the Anal Canal. Gastrointest Cancer Res 2013;6:39-45. [PubMed]
- Vieillot S, Azria D, Lemanski C, et al. Plan comparison of volumetric modulated arc therapy (RapidArc) and conventional intensity modulated radiation therapy (IMRT) in anal canal cancer. Radiat Oncol 2010;5:92. [Crossref] [PubMed]
- Stieler F, Wolff D, Lohr F, et al. A fast radiotherapy paradigm for anal cancer with volumetric modulated arc therapy (VMAT). Radiat Oncol 2009;4:48. [Crossref] [PubMed]
- Devisetty K, Mell LK, Salama JK, et al. A multi-institutional acute gastrointestinal toxicity analysis of anal cancer patients treated with concurrent intensity-modulated radiation therapy (IMRT) and chemotherapy. Radiother Oncol 2009;93:298-301. [Crossref] [PubMed]
- Han K, Cummings BJ, Lindsay P, et al. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int J Radiat Oncol Biol Phys 2014;90:587-94. [Crossref] [PubMed]
- Dasgupta T, Rothenstein D, Chou JF, et al. Intensity-modulated radiotherapy vs. conventional radiotherapy in the treatment of anal squamous cell carcinoma: a propensity score analysis. Radiother Oncol 2013;107:189-94. [Crossref] [PubMed]
- Chuong MD, Hoffe SE, Weber J, et al. Outcomes of anal cancer treated with definitive IMRT-based chemoradiation. J Radiat Oncol 2012;1:165-72. [Crossref]
- Franco P, Arcadipane F, Ragona R, et al. Volumetric modulated arc therapy (VMAT) in the combined modality treatment of anal cancer patients. Br J Radiol 2016;89:20150832. [Crossref] [PubMed]