|
Original Article
Survival of metastatic gastric cancer: Significance of age, sex
and race/ethnicity
Dongyun Yang1*, Andrew Hendifar2*, Cosima Lenz1, Kayo Togawa1, Felicitas Lenz2, Georg Lurje2, Alexandra Pohl2, Thomas Winder2, Yan Ning2, Susan Groshen1, Heinz-Josef Lenz1,2
1Department of Preventive Medicine, University of Southern California/Norris Comprehensive Cancer Center, Keck School of Medicine, Los Angeles, CA; 2Division of Medical Oncology, Sharon Carpenter Laboratory, University of Southern California/Norris Comprehensive Cancer Center, Keck School of Medicine, Los Angeles, CA
* Both authors contributed equally to this work.
This work was funded by the NIH grant P30 CA 14089, supported by the San Pedro Guild and the Dhont Foundation.
Corresponding to: Heinz-Josef Lenz, MD, FACP. Sharon A. Carpenter Laboratory, Division of Medical Oncology, University of Southern California, Norris Comprehensive Cancer Center, Keck School of Medicine, 1441 Eastlake Avenue, Los Angeles, CA 90033. Tel: +1-323-865-3967, Fax: +1-323-865-0061. Email: lenz@usc.edu.
|
|
Abstract
Background: Despite the success of modern chemotherapy in the treatment of large bowel cancers, patients with metastatic gastric cancer continue to have a dismal outcome. Identifying predictive and prognostic markers is an important step to improving current treatment approaches and extending survival.
Methods: Extracting data from the US NCI’s Surveillance, Epidemiology, and End Results (SEER) registries, we compared overall survival for patients with metastatic gastric cancer by gender, age, and ethnicity using Cox proportional hazards models. 13,840 patients (≥ 18 years) were identified from 1988-2004. Males and females were categorized by age grouping and ethnicity.
Results: 19% of Hispanic patients were diagnosed < 45 years of age as compared to 5.5% of Caucasians. Caucasian patients and men were more likely to be diagnosed with tumors in the gastric cardia (P<0.001). In our survival analysis, we found that women had a lower risk of dying as compared to men (P<0.001). Overall survival diminished with age (P<0.001). The median overall survival was 6 months in patients of ≤ 44 years old as compared to 3 months in patients 75 years and older. Gender differences in overall survival significantly varied by race and tumor grade/differentiation (P for interaction = 0.003 and 0.005, respectively).
Conclusion: This is the largest study of metastatic gastric cancer patients from the SEER registry to show that age, gender, and tumor location are significant independent prognostic factors for overall survival in patients with metastatic gastric cancer.
Key words
gastric cancer, gender, age, ethnicity, survival
J Gastrointest Oncol 2011; 2: 77-84. DOI: 10.3978/j.issn.2078-6891.2010.025
|
|
Introduction
Although its incidence and mortality has declined over the last half-century, gastric cancer remains the fourth
most common cancer and the second most frequent cause
of cancer death in the world ( 1, 2). The American Cancer
Society estimates that in 2008, there were 21,500 new cases
of gastric cancer and 10,880 deaths in the United States
( 3). As gastric cancer incidence declines, the frequency
of prox imal gastric and gastroesophageal junctional
adenocarcinomas continues to rise and has become a
significant clinical challenge ( 4, 5). There is substantial
geographic variation in the incidence and mortality of
gastric cancer, with the highest rates in East Asia and the
lowest in North America ( 2). H. pylori infection, dietary
factors, and smoking patterns may contribute to these
disparities ( 6-9). The survival rates for gastric cancer are among the worst of any solid tumor. Despite the success of modern
chemotherapy in the treatment of large bowel cancers, the
5-year survival of patients with advanced gastric cancer is
3.1% ( 1, 4). The role of surgery is also limited as only 23%
of stage IV gastric cancer patients receiving a palliative
gastrectomy are alive one year after surgery ( 4). Progress
was recently made as treating Her-2-Neu (H2N) overexpressing
gastric cancers with Traztuzumab was found to
significantly improve survival ( 10). Identifying additional
predictive and prognostic markers is an important step to
improving current treatment approaches and extending
survival. Two distinct histologic types of gastric cancer, the
“intestinal type” and “diffuse type”, have been described
( 11). The diffuse type of gastric cancer is undifferentiated
and characterized by the loss of E-cadherin expression; an
adhesion protein that helps maintains cellular organization
( 12). The well differentiated intestinal type is sporadic
and highly associated with environmental exposures,
especially H. pylori infection ( 13). There are also biologic
differences between these subtypes of gastric cancer that
may guide treatment approaches. H2N is over expressed
more often in the intestinal vs the diffuse type, 30% vs 6%
in one study ( 14). The Beta-catenin/Wnt signaling pathway
is also recognized to play a large role in the molecular
carcinogenesis of the intestinal type cancer ( 15). Despite the genetic heterogeneity of gastric cancer,
several biological determinants of risk and prognosis have
been identified. Genetic polymorphisms of cytokines
released with “oxidative stress” such as IL-Iβ, IL-10, and
TNF-A have been associated with increased gastric cancer
risk ( 16-18). Over expression of the oncogenes, tie-1, CMET
and AKT have been found to confer a poor prognosis in
both subtypes ( 19-21). Tumor expression of the isoenzyme
COX-2 is an independent prognostic factor for gastric
cancer survival ( 22). This benefit may be mediated by
a reduction in lymphangiogenesis, another correlate of
prognosis ( 22, 23). Recently Her-2/Neu over expression, an
important predictive and prognostic factor in breast cancer
has been independently associated with a poor prognosis in
gastric cancer ( 24, 25). The prognostic significance of age, gender, and ethnicity
in metastatic gastric cancer is unclear. The prevailing
belief that young patients with gastric cancer have a more
aggressive disease has been recently called into question
( 26, 27). Several prospective and population studies since
1996 have consistently shown that age is not a prognostic
factor for survival, despite the higher prevalence of “diffuse
type” cancer which typically has a worse outcome ( 28, 29).
However, according to a recent population-based study of
gastric cancer, a significant impact of age on survival was found in patients with stage IV disease ( 30). As compared to women, men are twice as likely to
develop and die from gastric cancer, in the US ( 1). Although
this may represent varying environmental exposures
between genders, studies demonstrate that menstrual
factors such as age of menopause and years of fertility are
associated with gastric cancer incidence ( 31). Interestingly,
woman may be more likely to have a “diffuse type histology”
( 32). There are also significant ethnic and racial differences
in gastric cancer incidence and survival. Asian patients
consistently have increased survival rates compared to
their western counterparts ( 33). Ethnic Asians living
in the US share this benefit which suggests that these
differences are not likely treatment related ( 34). Other
racial differences in the US are notable as the incidence
and mortality is 50% higher in African Americans than
Caucasians ( 35). Our study sought to evaluate the clinical correlates
of survival in metastatic gastric cancer. Specifically we
examined the inf luence of age, gender, ethnicity on
survival. We also explored the interactions between patient
characteristics and tumor histology, grade, size, and location
(cardia vs non-cardia).
|
|
Patients and methods
Data source
Adult patients with metastatic gastric cancer were identified
from the SEER registry 1988-2004 database, which collects
information on all new cases of cancer from 17 populationbased
registries covering approximately 26% of the US
population.
Study population
The disease was defined by the following International
Classification of Diseases for Oncology (ICD-O-2) codes:
C16.0-C16.9. We identified patients (n=15,360) who had
metastatic disease defined by SEER Extent of Disease code:
85. We restricted eligibility to adults (aged 18 years or
older) who were diagnosed with metastatic gastric cancer
(MGC) in 1988 and later (n=15,348); because the record of
extent of disease was not available for accurate staging prior
to 1988. We excluded cases (less than 10% of adult patients
with metastatic gastric cancer) who were diagnosed at death
certificate or autopsy, no follow-up records (survival time
code of 0 months), as well as lacking documentation on
race/ethnicity. A total of 13,840 MGC patients of 18 years
and older were included in the final sample for the current
analysis.
Variable definitions
Information on age at diagnosis, sex, race, and ethnicity,
marital status, treatment type, primary site, tumor grade
and differentiation, histology, tumor size, and lymph node
involvement, and overall survival were coded and available
in SEER database. The primary endpoint in this study was
overall survival that was defined as the months lapsing
from diagnosis to death. For the patients who were still
alive at last follow-up, overall survival was censored at the
date of last follow-up or December 31, 2004, whichever
came first.
Age. We chose the cut points for age groups based on the
previous studies (18-44, 45-54, 55-64, 65-74, and 75 and
older).
Ethnicity. Patients were divided into five ethnic groups,
“Caucasian” (Race/Ethnicity code, 1), “African American”
(Race/Ethnicity code, 2), “Asian” (Race/Ethnicity code,
4-97), “Hispanic” (Spanish/Hispanic Origin code, 1-8), and
Native American (Race/Ethnicity code, 3).
Primary site. According to the latest guidelines for
gastric cancer classificationa, the stomach is anatomically
delineated into the upper, middle, and lower thirds by
dividing the lesser and greater curvatures at two equidistant
points and joining these points. The sites were defined by
the following codes from ICD-O-2: Cardia, (C16.0), Body
(C16.1-2, C16.5-6), Lower (C16.3-4), and Overlapping
lesion of stomach (C16.8). For the ones that are not
specified, they were categorized together as Stomach, NOS
(C16.9).
Marital status. Subjects were categorized into “Not
married” (including never married, separated, divorced,
widowed, and unknowns) and “Married” (including
common law).
Treatment type. SEER variables, RX Summ-radiation
and RX summ-surg prim site were used to define treatment
types: “Surgery” for patients who had surgery (local
tumor destruction and excision, and gastrectomy) and/no
radiation, “Radiation therapy only” for patients who only
had radiation therapy, “Untreated” for patients who did
not have surgery nor radiation therapy, and “Unknown”.
Information on chemotherapy was not available in SEER.
Grade. Grade was defined by the following ICD-O-2
codes; well/moderately differentiated (Code 1-2), poorly
differentiated/undifferentiated (Code 3-4), and others
(Code 5-9).
Histological type. Histological types were defined by the
following ICD-O-3 codes: 8140- for adenocarcinoma, 8490
for Signet ring cell carcinoma, and the rest of the types were
categorized as ‘Others’.
The size of the primary tumor and the presence of
lymph node involvement were not of interest in the current analysis. Our cohort consisted entirely of patients with
metastatic disease.
Statistical analysis
Subjects were grouped by age to 18-44, 45-54, 55-64, 65-74,
and 75 and older. We stratified them by sex, race, marital
status, treatment type, grade, histological type, and primary
site. Descriptive statistics were calculated for categorical
variables using frequencies and proportions. Sex, race,
tumor grade, marital status, primary site, histological type,
and treatment type were independent variables. Differences
among age groups in each subgroup were evaluated using
the chi-square test.
We constructed Cox proportional hazards models to
examine the association between age and survival in men
and female separately. We compared survival across age
groups adjusting for potential confounders including
geographic region and year of diagnosis. By conducting
this ana lysis separately by gender, we were able to
determine pattern differences between genders. The Cox
proportional hazards model included year of diagnosis and
participating SEER registry site as stratification variables.
Marital status, treatment, primary site, histology, tumor
grade and differentiation, size of primary tumor, and lymph
node involvement were used as covariates. Hazard Ratios
(HRs) and 95% confidence intervals were generated, with
hazard ratios less than 1.0 indicating survival benefit (or
reduced mortality). Pairwise interactions (age and sex, age
and race, and sex and race) were checked using stratified
models and were tested by comparing corresponding
likelihood ratio statistics between the baseline and
nested Cox proportional hazards models that included
the multiplicative product terms ( 36). Departure of the
proportional hazard assumption of Cox models will be
examined graphically such as log-log survival curves or
smoothed plots of weighted Schoenfeld residuals ( 37) and
by including a time-dependent component individually for
each predictor. All analyses were conducted using P<0.05 as the
significance level and statistical analyses were performed
with the use of SAS software (version 9.1; SAS Institute,
Cary, NC).
|
|
Results
Patient baseline characteristics
The final cohort for analysis consisted of 13,840 patients,
8710 men (63%), and 5130 women (37%). Their age
distribution is as follows: 1,207 (9%) aged 18−44; 1,698
(12%) aged 45−54; 2,701 (20%) aged 55−64; 3,901 (28%)
aged 65−74; and 4,333 (31%) aged 75 years and older. The median age was 68 years (range: 18−104). 60% of the MGC
cohort were White, 13% African American, 13% Asian, 14 %
Hispanic, and 1% Native American. Tumor characteristics
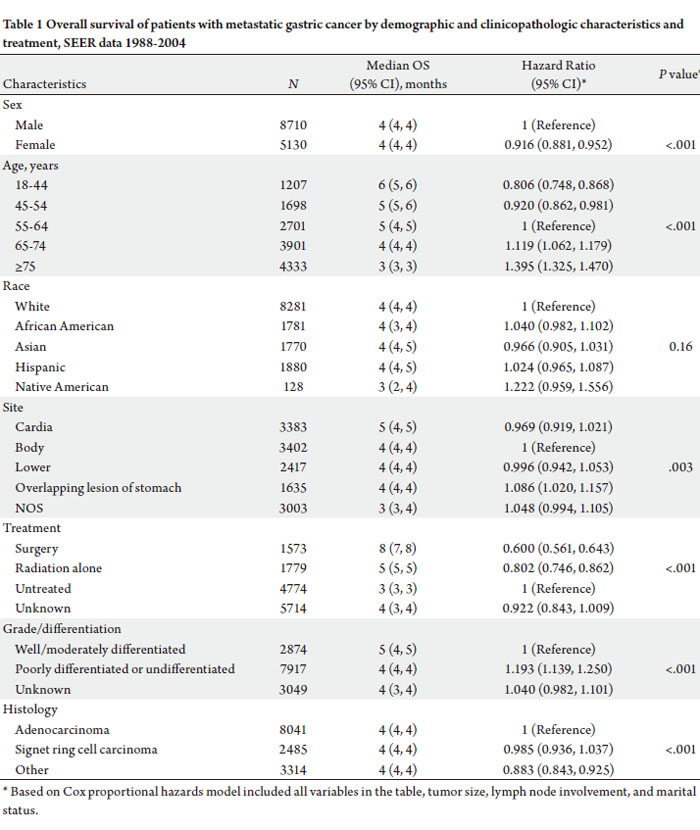
and treatment received are shown in Table 1.
Age and ethnicity in MGC
5.5 % of Whites with MGC were between 18-44 years of
ages as compared to 10% of African Americans, 11% of
Asians, and 19% of Hispanic patients. 36% of White gastric
cancer patients were diagnosed over 75 years of age; 29% of Asian, 27% of AA, and 20% of Hispanic.
Tumor location: cardia vs non-cardia
The incidence of cardia and non-cardia tumors varied
significantly depending on gender and ethnic background.
30% of men and 14% of women had gastric ca arising
from the cardia. The incidence of cardia cancers also
varied significantly across ethnicities. 32% of Whites had
cardia primaries, 13% of AA’s, 11% of Asians, and 14% of
Hispanics.
Survival analysis
The median overall survival (OS) in patients with MGC
was only 4 months. The prognostic significance of several
clinical and tumor characteristics were limited as the
median OS varied little when stratified by sex, race, tumor
site, grade/ differentiation, and histology (Table 1).
However, age, use of local treatment, tumor differentiation,
and tumor site were found to have a clinically significant
effect. The youngest group of patients had an improved OS
when compared to their older counterparts (Table 1), as the
median OS for patients 44 years or younger was 6 months
compared to 3 months in patients 75 years or older. Survival
was significantly worse in every successive age decile.
Patients who had received any treatment had significantly
improved survival. Gastrectomy or local surgery had a
median OS of 8 months compared to a median OS of 3
months in patients who were not treated with surgery or
radiation [HR = 0.600 (0.561, 0.643)] (Table 1). Similarly,
patients receiving radiation treatment had a survival benefit
[HR = 0.802 (0.746, 0.862)].
Tumor characteristics had a significant impact on
survival. As expected, patients with poorly differentiated
tumors had a worse survival than those with moderately
or well differentiated tumors [HR 1.19, P
In multivariate analysis, sex, age, treatment, and tumor
characteristics were significantly associated with overall
survival. Females had lower risk of dying compared to males
(HR=0.916, 95%CI: 0.881−0.952) and mortality increased
with age at diagnosis (P<0.001, Table 1). There was no
significant difference in OS across race/ethnicity groups
(P=0.16, Table 1).
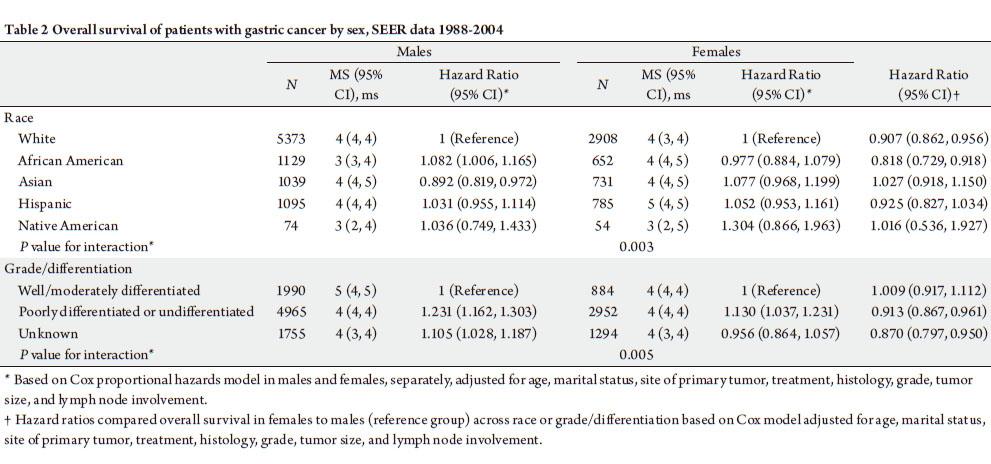
Sex, race, grade/differentiation and MGC
The effect of sex on OS was signif icantly varied by
race and tumor differentiation in patients with MGC
(Pfor interaction=0.003 and 0.005, respectively, Table 2).
White and African American woman had significantly lower risk of dying compared to their male counterparts. In
Asian, Hispanic, and Native American populations, men
and women had equivalent survival (Table 2.) Women also
had a significantly lower risk of dying compared to males
in patients whose tumors were poorly differentiated or
undifferentiated or had unknown tumor grade (Table 2).
|
|
Discussion
This cohort of metastatic gastric cancer patients from the
SEER database represents a wide cross-section of patients
with variable socioeconomic and ethnic backgrounds. Our
analysis also included a robust variety of pathology and is
likely a more generalizable representation than can be found
in clinical trials or case series.
As expected, we found tumor characteristics such as
grade, differentiation, and histology were associated with
survival in advanced gastric cancer. Notably, there was a
survival advantage attributable to gastric cardia lesions
when compared to non-cardia lesions. This sur vival
advantage persisted after controlling for the increased
prevalence of cardia lesions in Caucasians and men.
Survival differences between cardia and non-cardia
lesions may reflect differences in pathogenesis and tumor
biology. H. pylori infection is recognized as a unique risk
factor for non-cardia lesions while gastroesophageal reflux
disease plays a role in the development of proximal lesions
( 38, 39). Interestingly, there is growing evidence that H2N
expression is variably expressed in proximal and distal
gastric cancer lesions ( 40). The proto-oncogene Her-2/neu
(H2N) is located on chromosome 17q21 and encodes a
transmembrane tyrosine kinase growth factor receptor
featuring substantial homology with the EGFR ( 41, 42).
Over-expression of the H2N protein has been identified
in from 10 to 34% of breast cancers and is associated with
a poor prognosis ( 43). Over-expression of H2N has been
reported in gastric and gastro-esophageal tumors ( 24).
Additionally, there are studies describing H2N as a poor
prognostic factor in gastric cancer ( 40). Further studies are
needed to investigate its role in the development of proximal
and distal gastric lesions. In addition to tumor characteristics, patient features,
such as age and sex, also had significant prognostic impact.
Ethnicity – often described in gastric cancer literature
as having a prominent prognostic role – had no effect
on survival. We could not confirm previous reports that
Asian and Hispanic patients with gastric cancer have an
improved outcome. We did find that a higher percentage
of Hispanic patients present at a younger age. 36% of our
Hispanic patients presented at ages less than 54 yo vs 16%
of white patients. These findings are consistent with a single institution study, which found that
Hispanics present at a younger age when
compared to other ethnicities ( 44). After adjusting for sex, race, marital
status , treatment type, primary site,
histology, the year of diagnosis and SEER
site, we found significant increased cancerspecific
mortality among men and older age
groups. The survival for our youngest age
group was 2 fold higher than the oldest age
group. Our findings do not confirm previous
reports that younger patients with metastatic
gastric cancer have poorer survival. Outside
of treatment with surgery, young age was
the best prognostic marker. We could not
address the role of systemic chemotherapy
on overall survival in the current study due
to lack of information in SEER. This likely
ref lects the higher rate of treatment we
found in the younger patients and unlikely
represents differences in tumor biology or
kinetics.
Consistent with previous reports, we
found that women with MGC lived longer
than men. We did not find any association
between gender disparities and age.
Women of every age group, pre-and postmenopausal,
had an equivalent survival
advantage. When examined more closely,
we found that this difference was limited to
African American and White patients. There
were no gender differences in the Hispanic
and Asian patients. These differences were
not attributable to the presence of cardia
or non-cardia lesions. Although there have
been no reports of variable expression of
H2N by gender, there are gender differences
in expression of estrogen receptor (ER)
and ER messenger RNA in gastric cancer
( 45). A possible explanation for the survival
advantages in women may be found in a
recent study addressing the interactions
between the estrogen receptor and her-
2neu receptor pathways in breast cancer
development and treatment response.
Hurtado and colleagues found her-2-neu up
regulation following the silencing of PAX-2
in cell lines treated with tamoxifen, which
suggests that tamoxifen-estrogen receptor
and estradiol-estrogen receptor complexes
inhibits transcription of Her-2-Neu via Pax-2 ( 46). Despite the clinical and genetic variability of advanced
gastric cancer, we were able to identify clinical correlates
for improved outcomes, which included gender and age. We
did not find an association between ethnicity and survival.
This is thought provoking as there are clear differences
in the age of presentation and the prevalence of cardiac
tumors. Hispanic patients were twice as likely to develop
gastric cancer at < 45 years old than Caucasians. Conversely
Caucasians were twice as likely to develop gastric cardia
lesions vs non-proximal cancers. Further research into
biological basis for these differences is warranted.
|
|
References
- Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer.
Methods Mol Biol 2009;472:467-77.[LinkOut]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence,
mortality, and prevalence across five continents: defining priorities to
reduce cancer disparities in different geographic regions of the world. J
Clin Oncol 2006;24:2137-50.[LinkOut]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics,
2008. CA Cancer J Clin 2008;58:71-96.[LinkOut]
- Karpeh M, Kelsen D, Tepper J. Cancer of the stomach. In: DeVita
V, Hellman S, Rosenberg S, editors. Cancer Principles & Practice of
Oncology. Philadelphia: Lippincot, Williams & Wilkins; 2001. p.
1092-126.
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence
of esophageal and gastric carcinoma in the United States. Cancer
1998;83:2049-53.[LinkOut]
- Kamangar F, Qiao Y, Blaser MJ, Sun XD, Katki H, Fan JH, et al.
Helicobacter pylori and oesophageal and gastric cancers in a prospective
study in China. Br J Cancer 2007;96:172-6.[LinkOut]
- Bertuccio P, Praud D, Chatenoud L, Lucenteforte E, Bosetti C, Pelucchi
C, et al. Dietary glycemic load and gastric cancer risk in Italy. Br J
Cancer 2009;100:558-61.[LinkOut]
- Abnet CC, Freedman ND, Kamangar F, Leitzmann M, Hollenbeck AR,
Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric
and oesophageal adenocarcinomas: results from a cohort study and a
meta-analysis. Br J Cancer 2009;100:551-7.[LinkOut]
- Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Kubo M, et
al. Population-based prospective study of the combined inf luence of
cigarette smoking and Helicobacter pylori infection on gastric cancer
incidence: the Hisayama Study. Am J Epidemiol 2008;168:1409-15.[LinkOut]
- Van Custem E, Kang Y, Chung H, Shen L, Sawaki A, Lordick F, et al.
Efficacy results from the ToGA trial: A phase III study of trastuzumab
added to standard chemotherapy (CT) in first-line human epidermal
growth factor receptor 2 (HER2)-positive advanced gastric cancer
(GC) [abstract]. J Clin Oncol 2009;s27:LBA4509.
- Lauren P. The two histological main types of gastric carcinoma: diffuse
and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49.[LinkOut]
- Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P,
et al. E-cadherin germline mutations in familial gastric cancer. Nature
1998;392:402-5.[LinkOut]
- Takenaka R, Okada H, Kato J, Makidono C, Hori S, Kawahara Y, et
al. Helicobacter pylori eradication reduced the incidence of gastric
cancer, especially of the intestinal type. Aliment Pharmacol Ther
2007;25:805-12.[LinkOut]
- Vergara R, Torrazza I, Castillo Fernandez O. Her-2/Neu overexpression
in gastric cancer. J Clin Oncol 2009;s27:e15679.
- Clements W, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-
Preiser C, et al. beta-Catenin mutation is a frequent cause of Wnt
pathway activation in gastric cancer. Cancer Res 2002;62:3503-6.[LinkOut]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young
HA, et al. Interleukin-1 polymorphisms associated with increased risk
of gastric cancer. Nature 2000;404:398-402.[LinkOut]
- El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA,
Schoenberg JB, et al. Increased risk of noncardia gastric cancer
associated with proinf lammatory cytokine gene polymorphisms.
Gastroenterology 2003;124:1193-201.[LinkOut]
- Gorouhi F, Islami F, Bahrami H, Kamangar F. Tumour-necrosis
factor-A polymorphisms and gastric cancer risk: a meta-analysis. Br J
Cancer 2008;98:1443-51.[LinkOut]
- Amemiya H, Kono K, Itakura J, Tang RF, Takahashi A, An FQ, et al.
c-Met expression in gastric cancer with liver metastasis. Oncology
2002;63:286-96.[LinkOut]
- Cinti C, Vindigni C, Zamparelli A, La Sala D, Epistolato MC, Marrelli
D, et al. Activated Akt as an indicator of prognosis in gastric cancer.
Virchows Arch 2008;453:449-55.[LinkOut]
- Lin WC, Li AF, Chi CW, Chung WW, Huang CL, Lui WY, et al. tie-1
protein tyrosine kinase: a novel independent prognostic marker for
gastric cancer. Clin Cancer Res 1999;5:1745-51.[LinkOut]
- Mrena J, Wiksten JP, Thiel A, Kokkola A, Pohjola L, Lundin J, et al.
Cyclooxygenase-2 is an independent prognostic factor in gastric cancer
and its expression is regulated by the messenger RNA stability factor
HuR. Clin Cancer Res 2005;11:7362-8.[LinkOut]
- Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, et al.
Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via
reduction of lymphangiogenesis. Cancer Res 2007;67:10181-9.[LinkOut]
- Tanner M, Hollmén M, Junttila T, Kapanen A, Tommola S, Soini Y,
et al. Amplification of HER-2 in gastric carcinoma: association with
Topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol 2005;16:273-8.[LinkOut]
- Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, et al. HER-2/neu
amplification is an independent prognostic factor in gastric cancer. Dig
Dis Sci 2006;51:1371-9.[LinkOut]
- Holburt E, Freedman SI. Gastric carcinoma in patients younger than
age 36 years. Cancer 1987;60:1395-9.[LinkOut]
- Wang JY, Hsieh JS, Huang CJ, Huang YS, Huang TJ. Clinicopathologic
study of advanced gastric cancer without serosal invasion in young and
old patients. J Surg Oncol 1996;63:36-40.[LinkOut]
- Lee JH, Ryu KW, Lee JS, Lee JR, Kim CG, Choi IJ, et al. Decisions for
extent of gastric surgery in gastric cancer patients: younger patients
require more attention than the elderly. J Surg Oncol 2007;95:485-90.[LinkOut]
- Tso PL, Bringaze WL 3rd, Dauterive AH, Correa P, Cohn I Jr. Gastric
carcinoma in the young. Cancer 1987;59:1362-5.[LinkOut]
- Al-Refaie WB, Pisters PW, Chang GJ. Gastric adenocarcinoma in
young patients: A population-based appraisal [abstract]. J Clin Oncol
2007;s25:4547.
- Freedman ND, Chow WH, Gao YT, Shu XO, Ji BT, Yang G, et al.
Menstrual and reproductive factors and gastric cancer risk in a large
prospective study of women. Gut 2007;56:1671-7.[LinkOut]
- Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM.
Gastric adenocarcinoma: rev iew and considerations for future
directions. Ann Surg 2005;241:27-39.[LinkOut]
- Bollschweiler E, Boettcher K, Hoelscher AH, Sasako M, Kinoshita T,
Maruyama K, et al. Is the prognosis for Japanese and German patients
with gastric cancer really different? Cancer 1993;71:2918-25.[LinkOut]
- Theuer CP, Kurosaki T, Ziogas A, Butler J, Anton-Culver H. Asian
patients with gastric carcinoma in the United States exhibit unique
clinical features and superior overall and cancer specific survival rates.
Cancer 2000;89:1883-92.[LinkOut]
- Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, et
al. The annual report to the nation on the status of cancer, 1973-1997,
with a special section on colorectal cancer. Cancer 2000;88:2398-424.[LinkOut]
- Rothman K. Modern epidemiologyed. 2nd ed. Philadelphia: Lippincott-
Raven; 1998.
- Grambsch PM, Therneau TM. Propor tiona l hazards tests and
diagnostics based on weighted residuals. Biometrika 1994;81:512-26.[LinkOut]
- Lagergren J, Bergström R, Lindgren A, Ny rén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma.
N Engl J Med 1999;340:825-31.[LinkOut]
- Helicobacter and Cancer Collaborative Group. Gastric cancer and
Helicobacter pylori: a combined analysis of 12 case control studies
nested within prospective cohorts. Gut 2001;49:347-53.[LinkOut]
- Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the
gastrointestinal tract. Cancer Invest 2001;19:554-68.[LinkOut]
- Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The
product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein
with tyrosine kinase activity. Science 1986;232:1644-6.[LinkOut]
- Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath
J, et al. Tyrosine kinase receptor with extensive homology to EGF
receptor shares chromosomal location with neu oncogene. Science
1985;230:1132-9.[LinkOut]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire
WL. Human breast cancer: correlation of relapse and survival with
amplification of the HER-2/neu oncogene. Science 1987;235:177-82.[LinkOut]
- Yao JC, Tseng JF, Worah S, Hess KR, Mansfield PF, Crane CH, et al.
Clinicopathologic behavior of gastric adenocarcinoma in Hispanic
patients: analysis of a single institution's experience over 15 years. J Clin
Oncol 2005;23:3094-103.[LinkOut]
- Zhao XH, Gu SZ, Liu SX, Pan BR. Expression of estrogen receptor and
estrogen receptor messenger RNA in gastric carcinoma tissues. World J
Gastroenterol 2003;9:665-9.[LinkOut]
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson
RI, Brown M,et al. Regulation of ERBB2 by oestrogen receptor-PAX2
determines response to tamoxifen. Nature 2008;456:663-6.[LinkOut]
Cite this article as:
Yang D, Hendifar A, Lenz C, Togawa K, Lenz F, Lurje G, Pohl A, Winder T, Ning Y, Groshen S, Lenz H. Survival of metastatic gastric cancer: Significance of age, sex
and race/ethnicity. J Gastrointest Oncol. 2011;2(2):77-84. DOI:10.3978/j.issn.2078-6891.2010.025
|




