Endocuff assisted colonoscopy significantly increases sessile serrated adenoma detection in veterans
Introduction
In the United States, colorectal cancer (CRC) is the third most common cancer diagnosis among both men and women and the second leading cause of cancer related deaths in the United States (1,2). Colonoscopy prevents CRC with studies showing that the removal of adenomatous polyps reduces CRC mortality (3). Among the many colonoscopy quality measures, adenoma detection rate (ADR) has a significant impact on reducing CRC, with studies showing that every 1 percent increase in ADR decreases the risk for CRC by 3% (4,5).
Many accessories have been created to increase the detection of polyps and improve ADR. Among these the Endocuff overtube (MEDIVATORS) device has shown promise (6).
Our primary aim was to retrospectively compare ADRs and the distribution of polyp types with Endocuff assisted colonoscopy (EAC) and standard colonoscopy (SC) among a veteran population.
Methods
This study was designated as a Quality Improvement study by the Institutional Review Board (IRB) at the VA Loma Linda Health Care System (VALLHCS) and was approved by the IRB. We retrospectively reviewed patients who had received colonoscopies without Endocuff (EC) from January 6, 2014 through March 12, 2014 to compare to patients who underwent EAC from September 24, 2014 through February 19, 2015. Two staff endoscopists performed all procedures with experience using EC.
Patient selection
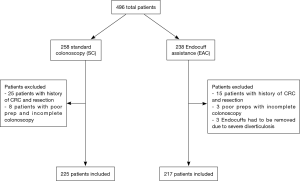
A total of 442 patients undergoing routine screening, surveillance and diagnostic colonoscopy were included in the study (Table 1). We excluded patients with prior resection for CRC, poor preparations leading to incomplete colonoscopy, colonic strictures, or severe diverticulosis leading to EC removal (Figure 1).
Full table
Endoscopic procedures
All patients in the Loma Linda VA system undergo split dose preparations with GOLYTELY (Braintree, New Jersey). Colonoscopies in both arms were performed with Olympus colonoscopes (190 series adult colonoscopes). Systems used for intraprocedural washing were the same for both endoscopists. The water exchange method was not used in either arm of the study. Patients were sedated either with moderate sedation using a combination of versed, fentanyl and Benadryl in a graded fashion to achieve adequate sedation, or with propofol in the presence of an anesthesiologist. A complete colonoscopy was defined as a colonoscopy that reached the cecum. The cecum was defined as identification of the ileocecal valve and the appendiceal orifice.
Measured outcomes
Statistical analysis was performed using SPSS (version 17), utilizing Chi-Squared and Fisher’s exact test statistical analysis. Primary outcomes were to ascertain if EAC improves ADR, and to identify the distribution of polyp types found, i.e., tubular adenomas and sessile serrated adenoma/polyps (SSA/P). We found a trend towards an increase in detection in the total number of SSA/Ps, and a SSA/P detection rate was calculated, defined as the percentage of colonoscopies that found at least one sessile SSA/P. Secondary outcomes were polyp detection rate (PDR), proximal colon ADR (cecum/ascending colon/hepatic flexure), distal colon ADR (transverse colon/splenic flexure/descending colon/sigmoid colon/rectum), cecal intubation rates (CIR), and complications between both groups.
Anatomy of the colon
The colon was divided into two segments, proximal and distal. The proximal colon was defined as the colon from the splenic flexure to the cecum. The distal colon was defined as the colon distal to the splenic flexure to the anal verge.
Pathology
Pathologic slides were reviewed by four anatomic pathologists at the VALLHCS in both groups and were not blinded. The same pathologists were involved during both time periods of the study. If discrepancies arose regarding pathologic diagnosis, especially SSA/P, a second opinion was obtained, and a consensus was put forth for diagnosis. If a hyperplastic polyp was diagnosed proximal to the splenic flexure, the endoscopist would ask to have the polyp re-evaluated to determine if the polyp was a SSA/P.
Results
Population/procedure characteristics
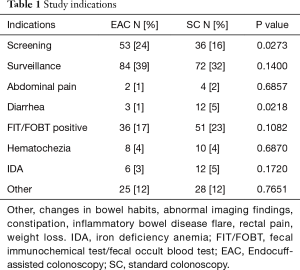
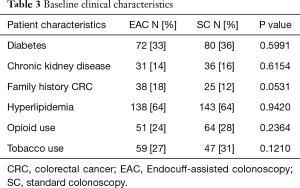
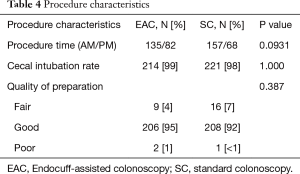
Out of the total population of 496 patients, 217 were included in the EAC group and 225 were in SC group (Figure 1). There was a male preponderance in both groups with a lower number of females in the SC group that did not reach statistical significance (P=0.0837). The median age for both groups was 65 years (Table 2). Both EAC and SC groups had similar percentages of comorbidities (hyperlipidemia, diabetes, tobacco use and CKD). The EAC group was found to have a slightly larger number of patients with a positive family history of CRC but was not found significant (P=0.0531) (Table 3). A higher percentage of EAC underwent colonoscopy for screening than in the SC group (P=0.0273), whereas diarrhea as an indication for colonoscopy was more predominant in the SC group (P=0.0218). All other indications were similar in both groups (Table 1). There was no statistical difference in the timing of the procedure (am/pm), and the preparation quality (P=0.387). CIRs were similar in both groups, EAC at 99% vs. SC at 98% (P=1.000) (Table 4). Unfortunately, withdrawal times were unable to be retrieved.

Full table
Full table
Full table
Polyp pathology
There were 59 SSA/P detected using EAC and only eight SSA/P using SC (Chi-square: 28.5665, 1; P value <0.0001). A similar number of tubular adenomas, tubulovillous adenomas, and adenocarcinomas were found in both groups (Table 5).
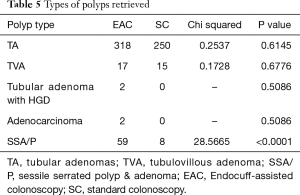
Full table
Complications
There were no reported complications related to EAC in our study period. The EC had to be removed in three cases due to severe diverticulosis (Table 6).
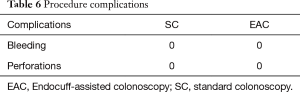
Full table
Detection rates
The SSA/P detection rate for EAC was 15% and for SC was 3%, accounting for an increase of 12% in the detection rates for SSA/P with EAC (P≤0.0001). The PDRs between EAC (72.81%) and SC (72.44%) were not significantly different (P=0.9311). The ADR for EAC was 59.91% and for SC was 50.66%. Although this accounted for a 9% increase in ADR when using EAC, the difference was found not to be statistically significant (P=0.0508). The proximal colon ADR for EAC was higher by 7% compared with SC, it was not found to be significantly different (P=0.0927). Lastly, the distal colon ADR for EAC was higher only by 2% (P=0.5834) (Table 7).
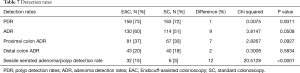
Full table
Pathologists
There were a total of four anatomic pathologists during the review period in both groups. No difference was seen between SC and EAC in regard to pathologist distribution (P=0.2546) (Table 8).
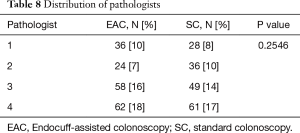
Full table
Discussion
This is the first study that demonstrates an increase in SSA/P detection with the use of EC in a veteran population. EAC was able to detect a total of 59 SSA/Ps compared to SC detecting eight (P≤0.0001), with a SSA/P detection rate of 15% with EAC and 3% with SC (P≤0.0001). Even though we cannot entirely attribute the increase in detection solely on EAC given the inherent limitations of our study, we believe that EAC played a significant role in the increased detection of SSA/Ps. The suspected mechanisms that EAC improved polyp detection included but were not limited to, detection of polyps behind haustral folds by flattening the folds exposing polyps, and removal of mucus cap debris.
Improved detection of SSA/Ps has been observed in two studies involving cap-assisted colonoscopy, one when cap assisted colonoscopy was coupled with a water exchange technique, and a second from a post hoc analysis of a prior study (7,8).
Sessile serrated adenomas/polyps, which are characterized by their serrated and distorted saw tooth appearance in colonic crypts (9) are felt to be a distinct class of pre-malignant adenoma, accounting for one third of all sporadic colorectal neoplasms (10). SSA/Ps are difficult to detect given their innate characteristics using standard devices (11). They tend to be flat with minimal mucosal aberrations, found primarily in the proximal colon, and often have a mucus cap with adherent debris concealing the lesion (11). Prevalence rates for SSA/Ps are in the range of 1% to 22% (12-14) but currently there is no consensus on recommended SSA/P detection rates. Interestingly the ADR does not include SSA/P detection, and may become a quality measure as we learn more about these lesions (13-15).
There are however recommended ADR targets. Increases in ADR are inversely correlated with CRC mortality and post colonoscopy CRC rates (4,14,16-19). These findings have led the American College of Gastroenterology Task Force on Quality in Endoscopy and the American Society for Gastrointestinal Endoscopy to update their ADR targets to >30% for men and >20% for women (20).
Since there has been a focus on ADR improvement, colonoscopic techniques, accessory technology and newer colonoscopes have been created to improve it. Some of these include: high-resolution endoscopic imaging, narrow-band endoscopic imaging, wide-angle colonoscopes, high definition colonoscopes, third eye retroscopes, Full Spectrum Endoscopy, balloon assisted colonoscopy (G-Eye), second forward view of the right colon and cecal retroflexion (19,21-24). The latest accessory devices in this field are the transparent cap colonoscopy (Endo-cap) and EC. These newer devices are simple, disposable, and relatively inexpensive attachments to the standard colonoscope. Both devices report higher ADRs, minimal patient discomfort, low complication rates, high CIRs and no additional procedure time (6,25). Our study was able to reproduce an increase in ADR of 9%, although it did not reach statistical significance. Further prospective studies will need to be performed to determine which setting and patient characteristics would be best to use accessory technology, like EC during colonoscopy.
There are strengths to our study. We found a significant increase in the total detection of SSA/Ps, as well as the detection rate when compared to SC, which has not been described with the use of EAC. Furthermore, although our ADR and proximal colon ADR did not reach statistical significance there was a trend towards improvement with ADR at 9% as seen on previous studies (6) and proximal colon ADR at 7%. This may be secondary to our sample size and experience of the endoscopists. Even though withdrawal technique were not reported, we feel EAC may have a played an important role. This modest increase in ADR and proximal colon ADR has important implications as it may alter surveillance intervals in patients with adenomatous polyps and serrated lesions. Lastly, we demonstrated that EAC is a safe and inexpensive method to help detect these polyps and can be incorporated into standard practice immediately.
There are limitations to the assessment. The study was uncontrolled and retrospective in design; in order to validate our results further prospective studies need to be conducted to show a suspected increased SSA/P detection rate for use in standard CRC screening populations. This study was conducted in a veteran population, with a male predominance and multiple predisposing risks for adenomatous polyps, which may not translate into the general population.
Another limitation was the lack of inclusion of withdrawal time, as this has been shown to influence ADR. However, there is some controversy on withdrawal time itself influencing ADR, with evidence to show the techniques used during withdrawal and the quality of the examination to play a more significant role in increasing ADR (26). Even though we did not include withdrawal time or the techniques used during withdrawal we felt that the quality of the colonoscopies was high given a high CIR (98%) and a high ADR with SC (51%). Other limitations of the study are that the pathologists were not blinded to the results of the colonoscopy given the retrospective nature, and patients from EAC and SC groups were obtained from two different time periods, which could potentially bias the findings.
There was also a slightly larger population of patients in our EAC group with a positive family history of CRC, which may have influenced the prevalence of adenomas. Finally, our study included screening, surveillance and diagnostic exams. Further prospective studies in a screening population will be needed to appropriately evaluate the true effectiveness of EAC.
In conclusion, we observed an increase in detection of SSA/P with the use of EAC in a veteran population and a trend in improving ADR. Further prospective studies will be needed to study the SSA/P detection rates and ADR with EAC specifically in a screening population.
Acknowledgements
None.
Footnote
Conflicts of Interest: Jackson CS is a consultant for Endocuff by MEDIVATORS. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was designated as a Quality Improvement study by the Institutional Review Board (IRB) at the VA Loma Linda Health Care System (VALLHCS) and was approved by the IRB (No. IORG0001204).
References
- Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290-314. [Crossref] [PubMed]
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 2009;22:191-7. [Crossref] [PubMed]
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095-105. [Crossref] [PubMed]
- Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298-306. [Crossref] [PubMed]
- Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362:1795-803. [Crossref] [PubMed]
- Floer M, Biecker E, Fitzlaff R, et al. Higher adenoma detection rates with endocuff-assisted colonoscopy—a randomized controlled multicenter trial. PLoS One 2014;9:e114267. [Crossref] [PubMed]
- Yen AW, Leung JW, Leung FW. A novel method with significant impact on adenoma detection: combined water-exchange and cap-assisted colonoscopy. Gastrointest Endosc 2013;77:944-8. [Crossref] [PubMed]
- Rzouq F, Gupta N, Wani S, et al. Cap assisted colonoscopy for the detection of serrated polyps: a post-hoc analysis. BMC Gastroenterol 2015;15:11. [Crossref] [PubMed]
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol 2008;32:21-9. [Crossref] [PubMed]
- Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315-29; quiz 1314, 1330.
- Sweetser S, Smyrk TC, Sinicrope FA. Serrated colon polyps as precursors to colorectal cancer. Clin Gastroenterol Hepatol 2013;11:760-7; quiz e54-5.
- East JE, Vieth M, Rex DK. Serrated lesions in colorectal cancer screening: detection, resection, pathology and surveillance. Gut 2015;64:991-1000. [Crossref] [PubMed]
- de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc 2013;77:617-23. [Crossref] [PubMed]
- Kahi CJ, Li X, Eckert GJ, et al. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc 2012;75:515-20. [Crossref] [PubMed]
- Lee CK, Kim YW, Shim JJ, et al. Prevalence of proximal serrated polyps and conventional adenomas in an asymptomatic average-risk screening population. Gut Liver 2013;7:524-31. [Crossref] [PubMed]
- Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533-41. [Crossref] [PubMed]
- Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol 2007;102:856-61. [Crossref] [PubMed]
- Kahi CJ, Anderson JC, Waxman I, et al. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterol 2010;105:1301-7. [Crossref] [PubMed]
- Chaptini L, Laine L. Can I improve my adenoma detection rate? J Clin Gastroenterol 2015;49:270-81. [Crossref] [PubMed]
- Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol 2006;101:873-85. [PubMed]
- Chandran S, Parker F, Vaughan R, et al. Right-sided adenoma detection with retroflexion versus forward-view colonoscopy. Gastrointest Endosc 2015;81:608-13. [Crossref] [PubMed]
- Dik VK, Moons LM, Siersema PD. Endoscopic innovations to increase the adenoma detection rate during colonoscopy. World J Gastroenterol 2014;20:2200-11. [Crossref] [PubMed]
- ASGE Technology Committee., Konda V, Chauhan SS, et al. Endoscopes and devices to improve colon polyp detection. Gastrointest Endosc 2015;81:1122-9. [Crossref] [PubMed]
- Kushnir VM, Oh YS, Hollander T, et al. Impact of retroflexion vs. second forward view examination of the right colon on adenoma detection: a comparison study. Am J Gastroenterol 2015;110:415-22. [Crossref] [PubMed]
- Rastogi A, Bansal A, Rao DS, et al. Higher adenoma detection rates with cap-assisted colonoscopy: a randomised controlled trial. Gut 2012;61:402-8. [Crossref] [PubMed]
- Lee RH. Quality colonoscopy: a matter of time, technique or technology? World J Gastroenterol 2013;19:1517-22. [Crossref] [PubMed]