Systematic review and a meta-analysis of hospital and surgeon volume/outcome relationships in colorectal cancer surgery
Introduction
In 1979, Luft et al. called for regionalisation of certain types of surgery after demonstrating a relationship between surgical volume and outcome (1). Although this was an intuitive observation implementation of the recommendations has been controversial. As recently as 2015 a consortia of hospitals in the US have pledged to meet minimum volume standards for certain surgery types including rectal cancer surgery (2).
Volume-outcome relationships have been studied with respect to surgeon volume, hospital volume and the interaction between each of these variables. In cancer surgery volume-outcome relationships may focus on measures of surgical safety such as morbidity and mortality, value and performance measures such as length of stay, and quality measures related to the oncologic outcome such as disease free and overall survival. An association between volume and outcomes has been reported for oesophagectomy (3,4), pancreatectomy (4) and hepatic resections (3).
Demonstrating surgeon or hospital volume-outcome relationships in colorectal cancer surgery has been unclear due to inconsistent results (5-8). Potential reasons for variation in the results could relate to the setting of the studies such as Medicare or Department of Veterans Affairs only, and geographical variation between states, countries and continents. In addition there might be variance depending on the site of surgery i.e. colon versus rectum, and type of surgery, open, laparoscopic or more recently robotic.
This review and meta-analysis aims to pool the current worldwide literature for the association between colorectal surgery outcomes with hospital volume and surgeon volume. This review will clarify several controversial issues including: (I) whether a hospital volume-outcome relationship exists in colorectal cancer resection; (II) whether a surgeon volume-outcome relationship exists; and (III) clarifying the association between high-hospital-volume-low-surgeon-volume versus low-hospital-volume-high-surgeon-volume.
Methods
Literature search
The present systematic review and meta-analysis was performed according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) (9), PRISMA guidelines (10) and recommendations (9). Electronic searches were performed using Embase, Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR) and ACP Journal Club and Database of Abstracts of Review of Effectiveness (DARE) from their dates of inception to May 2016. To achieve maximum sensitivity of the search strategy and identify all studies, the following terms were combined as either keywords of MeSH terms: “volume”, “outcome”, “colorectal surgery”, “colon surgery”, “rectal surgery”, “morbidity”, “mortality”, “complications”, “hospital” and/or “surgeon”. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria. Retrieval of the full articles of all available studies were conducted, including direct contact with authors.
Selection criteria
Eligible studies for the present systematic review and meta-analysis included those in which: (I) the subject of the study is the surgical treatment of colon, rectal or colorectal cancer; (II) hospital volume or surgeon volume is an independent variable tested; and (III) outcome parameters include post-operative mortality or survival. Studies that did not include mortality or complications as endpoints were excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. All publications were limited to those involving human subjects. There was no language restriction. Abstracts, unpublished studies, case reports, conference presentations, editorials, reviews and expert opinions were excluded.
Data extraction and appraisal
Two investigators independently conducted the study search [Y.R.H (BMed/MD, Honours), K.P. (MD, MPhil)]. The full articles of all relevant studies were extracted and evaluated for inclusion by both investigators. Discrepancies between the two reviewers were resolved by discussion and consensus with the two other authors [D.L.M (MD, PhD), W.L (MD)]. Study characteristics collected included year of publication, study period, primary country the study is based, population description, number of patients and number of hospitals. Outcomes extracted include 30-day mortality, in-hospital mortality, 5-year mortality, 5-year recurrence, local regional recurrence (LRR), postoperative complications, postoperative mortality, stoma rates, anastomic leak rates, and overall survival. Outcomes were extracted where possible as Hazard ratios (HR); 95% confidence interval (CI), and P values. Where possible, pooled outcomes were stratified by surgical procedure (rectal vs. colon vs. colorectal), and by the volume parameter (hospital procedure volume or surgeon volume).
Statistical analysis
Combined HRs for the association between hospital volume or surgeon volume with surgical outcomes were pooled under a random effects models. Heterogeneity analysis was performed using the Cochran Q test and I2 index. Computations were performed using Comprehensive Meta-Analysis v2 software (Biostat Inc, Englewood, New Jersey, USA).
Results
Methodologic characteristics of included studies
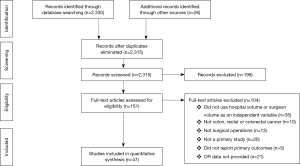
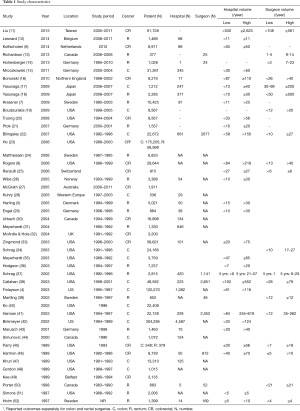
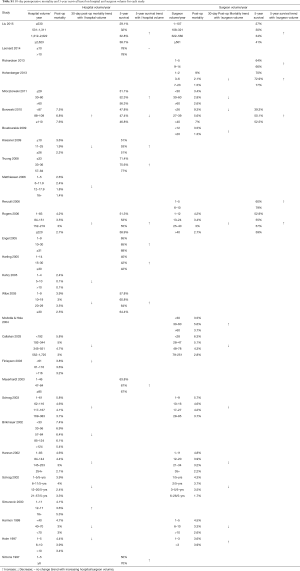
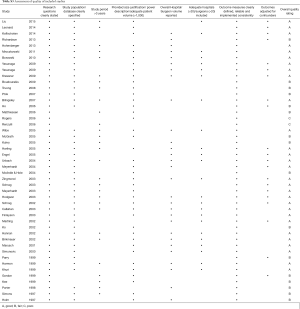
The initial search identified 2,330 potentially relevant articles to which the exclusion and inclusion criteria were applied (Figure 1). After exclusion of duplicate or irrelevant references, 2,315 potentially relevant articles were retrieved. After detailed evaluation of these articles, 151 studies remained for detailed assessment. After applying inclusion and exclusion criteria, 47 studies (Table 1) were included for the present systematic review and meta-analysis, of which: 18 only examined surgeon and/or hospital volume-outcome outcomes for rectal surgery, 15 for colon surgery, 13 for colorectal surgery, and 1 that reported outcomes for colon and rectal surgery separately (Table 1). Assessment of the quality of included studies are demonstrated in Table S1. Overall, 1,122,303 patients underwent colorectal surgery (in 9,877 hospitals by 9,649 surgeons).
Full table
Full table
The characteristics of each included study is summarized in Table 1. Each study stratified “high” and “low” volume groups to different cut-offs. A majority of studies determined their cut-offs based on their study sample and divided the numbers evenly between groups (i.e., median if two groups, quartiles if 4 groups). For “low” hospital volume, cut-offs ranged from 5 or less operation per 5 years (37) to 530 or less operations annually (11). For the “high” hospital volumes group, cut-off ranged from 6 or more annually (51) to 2,623 or more operations annually (11). For “low” surgeon volume, cut-offs ranged from 1 operation per 5 years (37), to 108 or less annually (11). For “high” surgeon volume, cut-offs ranged from 6–26 per 5 years (37), to 561 or more annually (11).
Mortality: 30-day, in-hospital and intra-operative
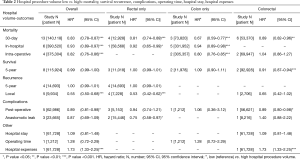
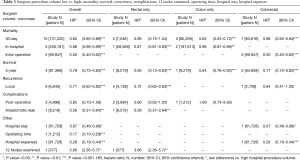
The association between mortality with hospital volume and surgeon volume is summarised in Table 2 and Table 3 respectively. Thirty day mortality was significantly lower in hospitals with higher procedure volume compared to lower volumes (HR: 0.83; 95% CI: 0.78–0.87, P<0.001). Subgroup analysis demonstrated that this association remained significant for rectal surgery alone (HR: 0.81; 95% CI: 0.74–0.89) and colon surgery alone (HR: 0.67; 95% CI: 0.59–0.77) (Table 2).
Full table
Full table
Similarly, surgeons with a higher procedure volume had a significantly lower 30-day mortality for colorectal surgery than surgeons with a lower volume (HR: 0.84; 95% CI: 0.80–0.89) (Table 3). Subgroup analysis demonstrated this association remained significant for colon surgery and colorectal surgery, but not for rectal surgery (Table 3).
In-hospital mortality was significantly lower in high volume hospitals compared to low volume hospitals (HR: 0.93; 95% CI: 0.89–0.97, P<0.001) (Table 2). Similarly, high volume surgeons had significantly lower in-hospital mortality than low volume surgeons (HR: 0.98; 95% CI: 0.96–0.99, P<0.001) (Table 3).
Intra-operative mortality was reported by two studies which demonstrated significantly lower rates in high volume hospitals (HR: 0.82; 95% CI: 0.76–0.86, P<0.001) (Table 2), and high level surgeons (HR: 0.50; 95% CI: 0.40–0.62, P<0.001) (Table 3) compared to their respective low counterparts.
Detailed post-operative mortality for each surgeon and/or hospital volume group is listed in Table S2.
Full table
Five-year survival
Five-year survival was significantly improved for high volume surgeons compared to low volume surgeons (HR: 0.79; 95% CI: 0.75–0.83, P<0.001) (Table 3). In comparison, 5-year survival was similar between low and high volume hospitals (HR: 0.99; 95% CI: 0.99–1.00). However, subgroup analysis demonstrated 5-year survival to be significantly better for high volume hospitals in the two studies which reported outcomes for colorectal surgery (HR: 0.91; 95% CI: 0.87–0.94, P<0.001) (Table 2). Detailed 5-year survival mortality for each surgeon and/or hospital volume group is listed in Table S2.
Local & 5-year recurrence
Local recurrence rates were reported in 5 studies (4 rectal and 1 colon surgery). Pooled results demonstrated local recurrence was significantly lower for high volume surgeons and high volume hospitals compared to low volume counterparts (HR: 0.71; 95% CI: 0.62–0.82, P<0.001 & HR: 0.55; 95% CI: 0.50–0.68, P<0.001 respectively) (Tables 2,3). Subgroup analysis demonstrated both associations were only significant for rectal surgery, not colon surgery. Five-year recurrence was similar for low and high volume hospitals (Table 2). No studies reported 5-year recurrence rates with surgeon procedure volume.
Post-operative complications
Compared to low-volume hospitals, high volume hospitals had significantly lower post-operative complications (HR: 0.89; 95% CI: 0.81–0.98, P<0.05) (Table 2). There was no significant difference in post-operative complications between high and low surgeon procedure volumes (HR: 0.85; 95% CI: 0.70–1.08) (Table 3).
Anastomotic leak
High procedure volume surgeons had significantly lower rates of anastomotic leak (HR: 0.59; 95% CI: 0.37–0.94, P<0.01) (Table 3). There were no differences in the incidence of anastomotic leaks between low and high volume hospitals (Table 2). However, subgroup analysis revealed that for rectal only resections, high volume hospitals had significantly lower rates of anastomotic leak compared to low volume hospitals (HR: 0.75; 95% CI: 0.58–0.97, P<0.05) (Table 2).
Trend between hospital/surgeon volume with 30-day mortality and 5-year survival
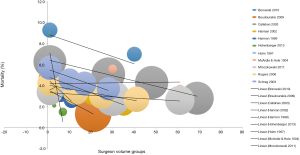
Rather than just “low” and “high” groups, a number of studies reported outcomes in three or more continuous volume groups to demonstrate a stepwise change in outcomes (Table S2). A total of 15 studies reported 30-day mortality rates for three or more consecutive increases in hospital volume. 30-day mortality was found to decrease with each stepwise increase in hospital volume in 87% (13/15) of studies (Figure 2). Furthermore, 5-year survival was found to increase with each stepwise increase in hospital volume in 91% (10/11) of studies (Table S2).
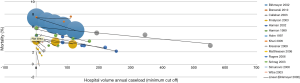
On the other hand, a total of 12 studies reported 30-mortality rates for three or more consecutive increases in surgeon volume. 30-day mortality was found to decrease with each stepwise increase in surgeon volume in 83% (10/12) of studies (Table S2, Figure 3). Furthermore, 5-year survival was found to increase with each stepwise increase in surgeon volume in 100% (6/6) of studies (Table S2).
Interaction between surgeon volume and hospital volume
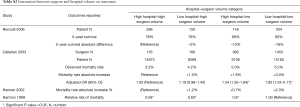
Four studies (25,38,41,46) examined the interaction between surgeon and hospital volume on outcomes. High-hospital-high-surgeon-volume had the best 5-year survival [78% in Renzulli et al. (25)] and lowest mortality rates [(3.2% in Callahan et al. (38)]. Low-hospital-high-surgeon-volume had better 5-year survival than high-surgeon-low-surgeon-volume [76% vs. 68% in Renzulli et al. (25)], and lower mortality rates in Callahan and colleagues’ study (14) (4.2% vs. 5.0%). However, one study (41) reported low-hospital-high-surgeon-volume had a higher mortality rate than high-surgeon-low-surgeon-volume (Table S3). Low-hospital-low-surgeon-volume had the highest mortality rate and lowest 5-year survival outcome in all four studies (Table S3).
Full table
Hospital stay
Liu et al. (11) [2015] identified hospital stay was significantly shorter with high volume surgeons whereby patients were more likely to be discharged within 14 days post-operatively (HR: 0.67; 95% CI: 0.46–0.99, P<0.05) (Table 3). Hospital volume was not associated with shorter hospital stay (Table 2).
Operating time
Yasunaga et al. (17) [2009] reported operating time was significantly reduced with high volume surgeons compared to low volume surgeons for colon surgery (HR: 0.17; 95% CI: 0.10–0.29, P<0.001) (Table 3). Hospital volume was not found to be associated with operating time (Table 2).
Hospital expenses
Liu et al. (11) [2015] defined hospital expenses as the total expenses during hospitalization and related expenditures for a definitive surgery. Interestingly, Liu and colleagues reported hospital expenses was higher with high volume hospitals (OR: 1.73; 95% CI: 1.33–2.25, P<0.001) (Table 2), but lower with high volume surgeons (OR: 0.29; 95% CI: 0.18–0.44, P<0.001) (Table 3).
Adequate lymph nodes resected
Richardson et al. (13) [2013] defined adequate lymph node resection as 12 lymph nodes in rectal surgery. The study reported high volume surgeons were more likely to have adequate lymph node resected compared to low volume surgeons (50% vs. 23% respectively, HR: 2.66; 95% CI: 2.36–5.70, P=0.01) (Table 3). No study examined the association between lymph node resection and hospital volume.
Discussion
This review and meta-analysis demonstrates that there is a volume-outcome relationship that favours high volume facilities and high volume surgeons. Higher hospital and surgeon volume resulted in reduced overall, in-hospital and intra-operative mortality. Post-operative complication rates depended on hospital not surgeon volume except with respect to anastomotic leak. Notably, high volume surgeons are associated with improved oncologic outcomes including greater lymph node retrieval, reduced recurrence rate and 5 year survival. Higher volume surgeons also improve value through reduced operative time, length of stay and cost. The best outcomes occur in high volume hospitals with high volume surgeons.
It is interesting that this meta-analysis demonstrates the rates of mortality are not the lowest in the studies with the highest hospital or surgeon annual case load. For example, studies where the highest volume had more than 100 operations annually than the lowest group had smaller reduction in mortality between groups than studies where the highest group had more than 20 operations annually than the lowest group. This makes it difficult to identify a clear threshold of effect, or a mathematical relationship between increasing volume and improvement in any of the outcome measures. A potential reason for this may be the highest volume hospitals have more surgeons, and therefore, each individual surgeon volume is low, whilst the smaller hospitals only have a few surgeons, and each individual surgeon volume is high. One study (41) reported low-hospital-high-surgeon-volume had a higher mortality rate than high-surgeon-low-surgeon-volume. However, three included studies demonstrated that low-hospital-high-surgeon-volume had better 5-year survival and lower risk of mortality than high-surgeon-low-surgeon-volume (25,38,46) (Table S3). This suggests that high surgeon volume is more critical than high hospital volume to optimise outcomes.
The mortality and 5-year survival varied between studies due to the different patient populations between and within the studies. The gold-standard for comparing surgical outcomes is to adjust for risk related to underlying patient characteristics that might influence the outcome (53). In the identified publications there was major variation in controlling for patient, cancer, hospital and surgeon characteristics. In addition, different risk measures such as Charlson score or ASA were used in different studies. Maruthappu et al. (53) has demonstrated overall poor methodological qualities for studies assessing individual surgical performance. Future studies should seek to control for patient characteristics and utilise risk stratification.
Numerous other factors may contribute to surgical outcomes. Some of these are directly related to volume, e.g., learning curve, and potentially explain the lack of continuous relationship between volume and outcome for surgeons or centres. It is worth noting that the annual volumes reported in this manuscript are often lower than those in studies of learning curve for colorectal surgery (54). This perhaps suggests that once initial learning curve is overcome then further improvement in outcomes is likely to be in small increments.
There has been a trend towards providing feedback to surgeons and physicians about their performance. It has been thought that feedback in its own right will lead to reflection and efforts to improve outcomes. This has been controversial particularly in the context where league tables are reported publically and linked to reimbursement. Recent studies comparing outcomes of hospitals participating in the American College of Surgeons’ National Surgical Quality Improvement Program (NSQIP) to non-NSQIP hospitals found no difference in rate of improvement in outcomes or in morbidity/mortality rates for a variety of general surgical procedures (55). Notably, however, there was across the board improvement overtime in both groups. Some suggest it may reflect a generally increased interest in quality improvement (55). However, as reasons for this are speculative, future research in this area is necessary to determine the most appropriate methods improve patient outcomes.
Volume outcome relationships have been challenged in other settings. Kurlansky et al. (56) demonstrate that low volume centres and low volume surgeons can have good outcomes if they are compliant with evidence-based quality standards (56). It is recognised that quality improvement requires multi-factorial interventions such as appropriate feedback and education (57). It may be that high volume for individual surgeons or high volume hospitals may actually be a surrogate for quality interventions such as protocolisation of care pathways, however future research to confirm this is warranted.
This study has limitations that need to be considered when interpreting the results. Firstly, there is little overlap between the cut-off points that define low and high volume groups between studies. As each health district and country holds different health service to population ratios, what is considered “high volume” is substantially different to another. This makes it difficult to demonstrate a universal cut-off level for minimum amount of operations annually to optimise outcomes. However, with most studies, the increase in surgeon and hospital volume is associated with a trend for reduced adverse outcomes, irrespective of absolute volume load. Secondly, some of the studies from USA used databases such as Medicare, SEER-Medicare, NCDB and California Cancer Registry which have overlapping patients. This may result in some patients being included multiple times in this meta-analysis. The studies from other countries such as Taiwan and Germany do not have this limitation as they are from independent hospital databases. Finally, heterogeneity exists between the studies in terms of the types of colorectal surgeries performed, distribution of cancer stages among hospitals and surgeons of various volumes. It is possible that those surgeons and hospitals with the highest volume perform more complex, high-risk operations. This would under-estimate the benefit of a high volume load over small volume load. Nevertheless, even with increasing volume loads, and the potential increase in more complex operations performed, a majority of the studies demonstrate a decrease on adverse outcomes with increasing volumes.
In conclusion, this study confirms that surgeon and hospital volume impacts colorectal surgical outcomes but fails to demonstrate a linear relation between volume and outcome. This suggests that whilst centralisation of such services may represent good policy, volume alone will not guarantee good or improved outcomes. Low and high volume surgeons and centres should seek to address quality through multiple methods, not just by increasing volume. Further studies on volume should better describe the quality improvement context of the operator such as participation in improvement programs, performance feedback and compliance with guidelines.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364-9. [Crossref] [PubMed]
- Urbach DR. Pledging to Eliminate Low-Volume Surgery. N Engl J Med 2015;373:1388-90. [Crossref] [PubMed]
- Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. [Crossref] [PubMed]
- Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg 2003;138:721-5; discussion 726. [Crossref] [PubMed]
- Harling H, Bülow S, Møller LN, et al. Hospital volume and outcome of rectal cancer surgery in Denmark 1994-99. Colorectal Dis 2005;7:90-5. [Crossref] [PubMed]
- Kolfschoten NE, Marang-van de Mheen PJ, Wouters MW, et al. A combined measure of procedural volume and outcome to assess hospital quality of colorectal cancer surgery, a secondary analysis of clinical audit data. PLoS One 2014;9:e88737. [Crossref] [PubMed]
- Kressner M, Bohe M, Cedermark B, et al. The impact of hospital volume on surgical outcome in patients with rectal cancer. Dis Colon Rectum 2009;52:1542-9. [Crossref] [PubMed]
- Rogers SO Jr, Wolf RE, Zaslavsky AM, et al. Relation of surgeon and hospital volume to processes and outcomes of colorectal cancer surgery. Ann Surg 2006;244:1003-11. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64.
- Liu CJ, Chou YJ, Teng CJ, et al. Association of surgeon volume and hospital volume with the outcome of patients receiving definitive surgery for colorectal cancer: A nationwide population-based study. Cancer 2015;121:2782-90. [Crossref] [PubMed]
- Leonard D, Penninckx F, Kartheuser A, et al. Effect of hospital volume on quality of care and outcome after rectal cancer surgery. Br J Surg 2014;101:1475-82. [Crossref] [PubMed]
- Richardson DP, Porter GA, Johnson PM. Surgeon knowledge contributes to the relationship between surgeon volume and patient outcomes in rectal cancer. Ann Surg 2013;257:295-301. [Crossref] [PubMed]
- Hohenberger W, Merkel S, Hermanek P. Volume and outcome in rectal cancer surgery: the importance of quality management. Int J Colorectal Dis 2013;28:197-206. [Crossref] [PubMed]
- Mroczkowski P, Kube R, Ptok H, et al. Low-volume centre vs high-volume: the role of a quality assurance programme in colon cancer surgery. Colorectal Dis 2011;13:e276-83. [Crossref] [PubMed]
- Borowski DW, Bradburn DM, Mills SJ, et al. Volume-outcome analysis of colorectal cancer-related outcomes. Br J Surg 2010;97:1416-30. [Crossref] [PubMed]
- Yasunaga H, Matsuyama Y, Ohe K, et al. Effects of hospital and surgeon volumes on operating times, postoperative complications, and length of stay following laparoscopic colectomy. Surg Today 2009;39:955-61. [Crossref] [PubMed]
- Yasunaga H, Matsuyama Y, Ohe K, et al. Volume-outcome relationship in rectal cancer surgery: a new perspective. Surg Today 2009;39:663-8. [Crossref] [PubMed]
- Boudourakis LD, Wang TS, Roman SA, et al. Evolution of the surgeon-volume, patient-outcome relationship. Ann Surg 2009;250:159-65. [Crossref] [PubMed]
- Truong C, Wong JH, Lum SS, et al. The impact of hospital volume on the number of nodes retrieved and outcome in colorectal cancer. Am Surg 2008;74:944-7. [PubMed]
- Ptok H, Marusch F, Kuhn R, et al. Influence of hospital volume on the frequency of abdominoperineal resection and long-term oncological outcomes in low rectal cancer. Eur J Surg Oncol 2007;33:854-61. [Crossref] [PubMed]
- Billingsley KG, Morris AM, Dominitz JA, et al. Surgeon and hospital characteristics as predictors of major adverse outcomes following colon cancer surgery: understanding the volume-outcome relationship. Arch Surg 2007;142:23-31; discussion 32. [Crossref] [PubMed]
- Ho V, Heslin MJ, Yun H, et al. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol 2006;13:851-8. [Crossref] [PubMed]
- Matthiessen P, Hallböök O, Rutegård J, et al. Population-based study of risk factors for postoperative death after anterior resection of the rectum. Br J Surg 2006;93:498-503. [Crossref] [PubMed]
- Renzulli P, Lowy A, Maibach R, et al. The influence of the surgeon's and the hospital's caseload on survival and local recurrence after colorectal cancer surgery. Surgery 2006;139:296-304. [Crossref] [PubMed]
- Wibe A, Eriksen MT, Syse A, et al. Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery at a national level. Br J Surg 2005;92:217-24. [Crossref] [PubMed]
- McGrath DR, Leong DC, Gibberd R, et al. Surgeon and hospital volume and the management of colorectal cancer patients in Australia. ANZ J Surg 2005;75:901-10. [Crossref] [PubMed]
- Kuhry E, Bonjer HJ, Haglind E, et al. Impact of hospital case volume on short-term outcome after laparoscopic operation for colonic cancer. Surg Endosc 2005;19:687-92. [Crossref] [PubMed]
- Engel J, Kerr J, Eckel R, et al. Influence of hospital volume on local recurrence and survival in a population sample of rectal cancer patients. Eur J Surg Oncol 2005;31:512-20. [Crossref] [PubMed]
- Urbach DR, Baxter NN. Does it matter what a hospital is "high volume" for? Specificity of hospital volume-outcome associations for surgical procedures: analysis of administrative data. Qual Saf Health Care 2004;13:379-83. [Crossref] [PubMed]
- Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of hospital procedure volume on surgical operation and long-term outcomes in high-risk curatively resected rectal cancer: findings from the Intergroup 0114 Study. J Clin Oncol 2004;22:166-74. [Crossref] [PubMed]
- McArdle CS, Hole DJ. Influence of volume and specialization on survival following surgery for colorectal cancer. Br J Surg 2004;91:610-7. [Crossref] [PubMed]
- Zingmond D, Maggard M, O'Connell J, et al. What predicts serious complications in colorectal cancer resection? Am Surg 2003;69:969-74. [PubMed]
- Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol 2003;83:68-78; discussion 78-9. [Crossref] [PubMed]
- Meyerhardt JA, Catalano PJ, Schrag D, et al. Association of hospital procedure volume and outcomes in patients with colon cancer at high risk for recurrence. Ann Intern Med 2003;139:649-57. [Crossref] [PubMed]
- Hodgson DC, Zhang W, Zaslavsky AM, et al. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst 2003;95:708-16. [Crossref] [PubMed]
- Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg 2002;236:583-92. [Crossref] [PubMed]
- Callahan MA, Christos PJ, Gold HT, et al. Influence of surgical subspecialty training on in-hospital mortality for gastrectomy and colectomy patients. Ann Surg 2003;238:629-36; discussion 636-9. [PubMed]
- Martling A, Cedermark B, Johansson H, et al. The surgeon as a prognostic factor after the introduction of total mesorectal excision in the treatment of rectal cancer. Br J Surg 2002;89:1008-13. [Crossref] [PubMed]
- Ko CY, Chang JT, Chaudhry S, et al. Are high-volume surgeons and hospitals the most important predictors of in-hospital outcome for colon cancer resection? Surgery 2002;132:268-73. [Crossref] [PubMed]
- Hannan EL, Radzyner M, Rubin D, et al. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery 2002;131:6-15. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Marusch F, Koch A, Schmidt U, et al. Hospital caseload and the results achieved in patients with rectal cancer. Br J Surg 2001;88:1397-402. [Crossref] [PubMed]
- Simunovic M, To T, Baxter N, et al. Hospital procedure volume and teaching status do not influence treatment and outcome measures of rectal cancer surgery in a large general population. J Gastrointest Surg 2000;4:324-30. [Crossref] [PubMed]
- Parry JM, Collins S, Mathers J, et al. Influence of volume of work on the outcome of treatment for patients with colorectal cancer. Br J Surg 1999;86:475-81. [Crossref] [PubMed]
- Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 1999;230:404-11; discussion 411-3. [Crossref] [PubMed]
- Khuri SF, Daley J, Henderson W, et al. Relation of surgical volume to outcome in eight common operations: results from the VA National Surgical Quality Improvement Program. Ann Surg 1999;230:414-29; discussion 429-32. [Crossref] [PubMed]
- Gordon TA, Bowman HM, Bass EB, et al. Complex gastrointestinal surgery: impact of provider experience on clinical and economic outcomes. J Am Coll Surg 1999;189:46-56. [Crossref] [PubMed]
- Kee F, Wilson RH, Harper C, et al. Influence of hospital and clinician workload on survival from colorectal cancer: cohort studyCommentary: How experienced should a colorectal surgeon be? BMJ 1999;318:1381. [Crossref] [PubMed]
- Porter GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg 1998;227:157-67. [Crossref] [PubMed]
- Simons AJ, Ker R, Groshen S, et al. Variations in treatment of rectal cancer: the influence of hospital type and caseload. Dis Colon Rectum 1997;40:641-6. [Crossref] [PubMed]
- Holm T, Johansson H, Cedermark B, et al. Influence of hospital- and surgeon-related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. Br J Surg 1997;84:657-63. [Crossref] [PubMed]
- Maruthappu M, El-Harasis MA, Nagendran M, et al. Systematic review of methodological quality of individual performance measurement in surgery. Br J Surg 2014;101:1491-8; discussion 1498. [Crossref] [PubMed]
- Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 2011;25:855-60. [Crossref] [PubMed]
- Etzioni DA, Wasif N, Dueck AC, et al. Association of hospital participation in a surgical outcomes monitoring program with inpatient complications and mortality. JAMA 2015;313:505-11. [Crossref] [PubMed]
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg 2012;143:287-93. [Crossref] [PubMed]
- Scheinerman SJ, Dlugacz YD, Hartman AR, et al. Journey to top performance: a multipronged quality improvement approach to reducing cardiac surgery mortality. Jt Comm J Qual Patient Saf 2015;41:52-61. [Crossref] [PubMed]