Bone marrow tolerance during postoperative chemotherapy in colorectal carcinomas
Introduction
Colorectal cancer in the United States is expected to account for approximately 93,000 new cases of colon cancer and 40,000 of rectal cancer in 2016 (1). For stage III colon cancer, surgical resection followed by adjuvant chemotherapy consisting of 5-Flurouracil (5-FU) and oxaliplatin based chemotherapy (OxF) is the preferred treatment strategy (2,3). This is in contrast to the management of locally advanced rectal cancer (T3/T4 or lymph node positive) which consists of preoperative chemoradiation therapy (CRT) (4) followed by total mesorectal excision, and often postoperative chemotherapy consisting of OxF (5).
There have been several studies evaluating the relationship between incidental pelvic bone marrow (PBM) irradiation and hematologic toxicity (HT) during CRT (6,7) in an attempt to create radiotherapy dose constraints to the PBM. Since nearly 40% of total human bone marrow (BM) (8) is in the PBM, sparing of the BM is thought to limit morbidity during the short period of CRT. However, in reality, irradiating bone marrow (BM) is likely to result in long term myelosuppression since there can be injury to BM stem cells (9) as well as declines in stromal volume which provide stimulating factors crucial for normal hematopoiesis (9).
Since oxaliplatin itself is myelosuppressive, rectal cancer patients receiving adjuvant/consolidative OxF chemotherapy may experience difficulty tolerating this regimen given the prior PBM stress endured during CRT. This may explain why rates of ≥grade 3 HT (HT3) for rectal cancer patients during CRT are 10% (10) and nearly 40% during postoperative OxF therapy (5). Interestingly colon cancer patients, who do not receive prior CRT, experience similar rates of HT3 of nearly 35%, and those above the age of 70 experience rates as high as 40% during adjuvant therapy (11).
However, what is not well reported in the literature is the timing of when rectal cancer patients experience HT3 compared to colon cancer patients or whether they require more dose reductions during adjuvant chemotherapy.
This study sought to compare the rates and timing of HT3 during postoperative chemotherapy in rectal cancer patients, who receive preoperative chemoradiation vs. colon cancer patients who do not. We hypothesized that rectal cancer patients would experience more instances of HT3 at earlier time points compared to colon cancer patients who do not receive preoperative CRT. The secondary endpoint of this study was to evaluate the rate and timing of a hematologic event (HE) defined as a ≥grade 2 HT along with a dose reduction or missed dose of chemotherapy.
Methods
Patient inclusion criteria
After Institutional Review Board (IRB) approval, we conducted a retrospective study of rectal cancer patients who were treated with preoperative CRT at this institution (12) and colon cancer patients treated with postoperative chemotherapy. Patients’ chemotherapy during CRT must have consisted of capecitabine or infusional 5-FU. Patients who received concurrent oxaliplatin during CRT were excluded. Postoperative chemotherapy must have consisted of OxF (modified FOLFOX-6 or CapeOx) resulting in 35 patients. No prospective trials have demonstrated differences in HT of CapeOx compared to modified FOLFOX-6 (13). Three rectal cancer patients and one colon cancer patient with oligometastatic disease were included, as these patients were treated with curative intent and with a similar number of chemotherapy cycles. Furthermore, additional treatment with bevacizumab does not alter the HT profile in addition to FOLFOX (14). Colon cancer patients must have been treated with OxF adjuvant chemotherapy after surgery (Stage III), with available weekly complete blood cell (CBC) counts leading to 42 patients.
Radiation treatment planning
Rectal cancer patients underwent radiation treatment consistent with Radiation Therapy Oncology Group treatment guidelines for rectal cancers (12,15,16). Patients were treated with either intensity-modulated radiation therapy (IMRT) (n=20, 57.1%) or 3D-conformal radiation therapy (3D-CRT) (n=15, 42.8%) to a median dose of 50.4 Gy (range: 48.6–54 Gy) in 1.8 Gy/day fractions. Patients receiving Intensity Modulated Radiation Therapy (IMRT) were treated as per RTOG 0822 with additional institutional dose constraints consisting of the iliac bone marrow (IBM) and femoral head volume receiving 30 Gy <50% (17,18). Similarly, the median volume receiving 30 Gy to the IBM for rectal cancer patients in this study was 37%.
Chemotherapy delivery
Rectal cancer patients during neoadjuvant CRT were treated with capecitabine (825 mg/m2 orally twice daily) or with 5-FU (225 mg/m2/day). All patients received postoperative adjuvant OxF, primarily with modified FOLFOX-6 protocol consisting of Folinic acid (400 mg/m2) and oxaliplatin (85 mg/m2) on day 1, 5-Fluoruracil (400 mg/m2) on day 1 followed by 2,400 mg/m2 over 46 hours in two week cycles for up to 12 cycles. For Rectal cancer patients, the adjuvant chemotherapy duration was determined by subtracting days on CRT from total planned days of adjuvant treatment. Colon cancer patients were also treated in a similar manner with modified FOLFOX-6. Four rectal cancer patients and one colon cancer patient received CapeOx as adjuvant treatment given as capecitabine (850 mg/m2 orally twice daily) and Oxaliplatin (130 mg/m2 on day one) for up to 8 cycles every 3 weeks. No prospective trials have demonstrated differences in HT of CapeOx compared to modified FOLFOX-6 (13).
Hematologic toxicity
Both colon cancer and rectal cancer patients had weekly CBCs during postoperative chemotherapy. HT3 was graded according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0. HE was defined as HT3 or ≥grade 2 HT plus a dose reduction or missed dose of adjuvant OxF therapy as a direct result of the HT.
Statistical analysis
The Shapiro-Wilk test was first done to test for normal data distribution. Categorical variables were described as the absolute number and percentage, and continuous variables were described by the median and interquartile ranges (IQR) due to right-skewed distribution. Chi-square tests and Fisher’s exact test (if expected frequencies were <5) were used to analyze categorical data.
Kaplan-Meier curves and log rank test were used to estimate and compare HT3-free and HE-free progression during adjuvant therapy in rectal cancer vs. colon cancer. Patients were censored at the last day of completion of adjuvant chemotherapy. Cox regression analysis was done to assess the hazard ratio of experiencing HT3. All p values were 2-sided with a level <0.05 considered as significant. All statistics and graphs were calculated and created using R software 3.2.0 (Vienna, Austria, http://www.R-project.org).
Results
Patient characteristics
Patients’ baseline characteristics are detailed in Table 1. There were 35 rectal cancer patients evaluated with a median age of 53 years, the majority being males (80%). Most patients had node positivity found during clinical staging (72%). During CRT, patients were mostly treated with IMRT (57.1%) and capecitabine (82.9%). During postoperative OxF chemotherapy, rectal cancer patients received FOLFOX (83%), FOLFOX/Bevacizumab (8.6%) and CapeOx (8.6%). There were 42 colon cancer patients evaluated with a median age of 60.5 years, and 52.4% were males. All colon cancer patients had node positive disease on pathological staging and the majority were treated with FOLFOX only (95.2%) with one patient receiving CapeOx (2.4%) and one receiving FOLFOX with bevacizumab (2.4%).
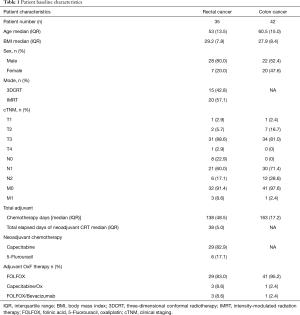
Full table
Hematologic toxicity during postoperative chemotherapy
Table 2 reveals that during adjuvant chemotherapy for rectal cancer, 40% (n=14) of patients experienced HT3 compared to 26.1% (n=11) in the colon cancer group (P=0.4). HE occurred in 48% (n=17) of rectal cancer patients versus 36% (n=15) in colon cancer group, (P=0.36). Rectal cancer patients mainly experienced HT3 consisting of leukopenia 25.7% (n=9), neutropenia 20.0% (n=7), and thrombocytopenia 2.9% (n=1) while none were due to anemia. There were 11.4% (n=4) who experienced both HT3 leukopenia and neutropenia. In the colon cancer group, there were 9.5% (n=4) patients who had HT3 leukopenia, 9.5% (n=4) had neutropenia, and 7.1% (n=3) had anemia, with 4.8% (n=2) having both leukopenia and neutropenia. There were 25.8% (n=9) of rectal cancer patients that required pegfilgrastim support during adjuvant chemotherapy compared to 11.9% (n=5) in colon cancer group (P=0.20).
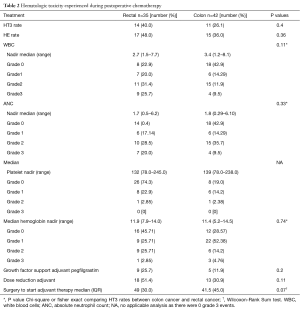
Full table
Clinical parameters
Table 3 describes the association between clinical parameters (age, BMI, gender, total time from surgery until adjuvant therapy) and HT3/HE for rectal cancer patients and colon cancer patients. Interestingly, no clinical parameters, including total time from surgery until adjuvant therapy seemed to have associated with HT3 or with HE.
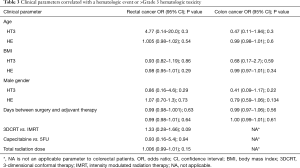
Full table
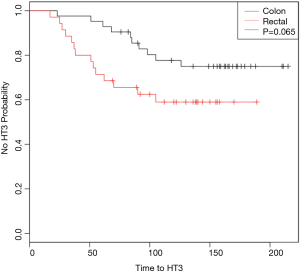
Time to ≥ grade 3 hematologic toxicity
Figure 1 demonstrates Kaplan-Meier curves that show probability of being free of HT3 over the course of treatment. Log-rank analysis revealed a p value of 0.065. Univariate Cox regression revealed the hazard ratio (HR) to be 1.8 (0.95–3.75, P=0.07). Multivariate Cox regression analysis, when adjusting for total days from surgery until starting chemotherapy, gender, and age demonstrated that rectal cancer patients were at an increased risk of HT3 (HR =2.49; 95% CI: 1.02–6.02, P=0.045).
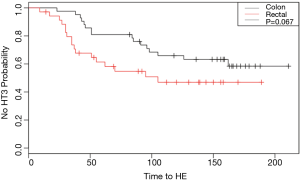
Time to hematologic event
Figure 2 demonstrates a Kaplan-Meier curve showing the probability of being HE-free during the course of OxF treatment. Log-rank analysis demonstrates a P value of 0.067. Univariate Cox regression analysis demonstrated the HR to be 1.8 (95% CI: 0.94–3.5, P=0.07). Multivariate Cox regression analysis adjusting for total days from surgery until starting chemotherapy, gender, and age demonstrated a HR of 2.07 (95% CI: 0.99–4.3, P=0.053).
Discussion
In this patient sample, we were able to demonstrate that rectal cancer patients experience HT3 sooner on multivariate analysis while adjusting for imperative covariates. We believe that the earlier timing of hematologic complications in rectal cancer is a reflection of underlying incidental injury to the pelvic bone marrow from prior radiation therapy. This has clinical implications for not only more cautious sparing of the PBM during CRT but also suggests increased hematologic morbidity amongst rectal cancer patients. Although pegfilgrastim use was not statistically significantly greater when used in rectal cancer patients compared to colon cancer, its use was nearly doubled in the rectal cancer group compared to colon cancer. These findings underscore the importance of PBM sparing during CRT as durable injury to the BM may make the added stress of cytotoxic chemotherapy less tolerable. We suspect though given a larger sample size this would become more evident, given the literature suggesting decreased marrow reserve after irradiation.
Radiation to the PBM had been implicated in diminishing long term BM function. During CRT there is injury to quiescent BM stem cells and stromal supporting elements (19) when examining in vitro cells (CD34+) and human bone marrow (20,21). A recent study examined patients treated with capecitabine and radiation for rectal cancer (similar to our cohort) and found that there are decreases to the mean proton density fat fraction (PDFF), crucial for supporting hematopoiesis, when comparing MRIs before and after CRT (22). For these two reasons, the BM may have less marrow reserve to tolerate ensuing insults (19,23,24). There are no uniform tables to guide clinicians about pelvic bone marrow sparing, as the bone marrow was not allotted dose constraints in the original normal tissue toxicity from radiation tables by Emami (25).
While we found higher rates of HT3 and HE in rectal cancer patients compared to colon cancer patients, we were unable to demonstrate so with statistical significance. We believe though that our sample was underpowered (mainly due to strict inclusion criteria to ensure homogeneity) to demonstrate the differences in rates of HT3 and HE although we suspect rectal cancer patients experience these events in greater numbers compared to colon cancer.
This study had several strengths, namely homogeneity amongst patient samples. In order to ensure that the long-term suppression was mainly due to the pelvic RT, this study included rectal cancer patients only treated with capecitabine or continuous infusion 5-FU as part of the CRT regimen since these have similar toxicity (26) and mainly function as radiosensitizers (27,28). By including colon cancer patients treated with oxaliplatin in the adjuvant treatment period, we sought to control for the extent of expected chemotherapy induced BM injury to best ascertain the importance of dosimetric sparing during CRT.
There were some limitations to this study. Besides limitations inherent to retrospective analysis, there are limitations in that the effects of 5-FU versus capecitabine on the long-term function of pelvic BM are unknown, although evidence suggests they are mainly radiosensitizers. Since oxaliplatin is myelosuppressive, we did not include any rectal cancer patients treated during neoadjuvant CRT with oxaliplatin. Furthermore while bevacizumab does not cause added toxicity (14), there are no trials, which compare FOLFOX to CapeOX chemotherapy in the adjuvant setting in colorectal cancer since both are recommended agents (29). Ultimately though, the comparison with colon cancer patients created a control in this retrospective study and helped to better demonstrate the longer term effects of BM suppression during CRT in rectal cancer patients. Ideally, the rates of HT and the timing before experiencing HT should be evaluated in a prospective manner.
Conclusions
Rectal cancer patients are more likely to experience HT3 earlier than colon cancer patients. When presented with patients with rectal cancer who are likely to receive OxF based adjuvant chemotherapy, clinicians should consider sparing the PBM from radiation during preoperative neoadjuvant CRT which may make adjuvant chemotherapy more tolerable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics board of Rutgers Cancer Institute of New Jersey (NO. Pro20140001136).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768-74. [Crossref] [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 2014;15:1245-53. [Crossref] [PubMed]
- Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2011;79:800-7. [Crossref] [PubMed]
- Yang TJ, Oh JH, Apte A, et al. Clinical and dosimetric predictors of acute hematologic toxicity in rectal cancer patients undergoing chemoradiotherapy. Radiother Oncol 2014;113:29-34. [Crossref] [PubMed]
- ELLIS RE. The distribution of active bone marrow in the adult. Phys Med Biol 1961;5:255-8. [Crossref] [PubMed]
- Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1319-39. [Crossref] [PubMed]
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [Crossref] [PubMed]
- Hamza S, Bouvier AM, Rollot F, et al. Toxicity of oxaliplatin plus fluorouracil/leucovorin adjuvant chemotherapy in elderly patients with stage III colon cancer: a population-based study. Ann Surg Oncol 2014;21:2636-41. [Crossref] [PubMed]
- Newman NB, Sidhu MK, Baby R, et al. Long-Term Bone Marrow Suppression During Postoperative Chemotherapy in Rectal Cancer Patients After Preoperative Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2016;94:1052-60. [Crossref] [PubMed]
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. [Crossref] [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [Crossref] [PubMed]
- Jabbour SK, Patel S, Herman JM, et al. Intensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visits. Int J Surg Oncol 2012;2012:891067.
- Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009;74:824-30. [Crossref] [PubMed]
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [Crossref] [PubMed]
- Hong TS, Moughan J, Garofalo MC, et al. NRG Oncology Radiation Therapy Oncology Group 0822: A Phase 2 Study of Preoperative Chemoradiation Therapy Using Intensity Modulated Radiation Therapy in Combination With Capecitabine and Oxaliplatin for Patients With Locally Advanced Rectal Cancer. Int J Radiat Oncol Biol Phys 2015;93:29-36. [Crossref] [PubMed]
- Mauch P, Lamont C, Neben TY, et al. Hematopoietic stem cells in the blood after stem cell factor and interleukin-11 administration: evidence for different mechanisms of mobilization. Blood 1995;86:4674-80. [PubMed]
- Drouet M, Mathieu J, Grenier N, et al. The reduction of in vitro radiation-induced Fas-related apoptosis in CD34+ progenitor cells by SCF, FLT-3 ligand, TPO, and IL-3 in combination resulted in CD34+ cell proliferation and differentiation. Stem Cells 1999;17:273-85. [Crossref] [PubMed]
- FitzGerald TJ, McKenna M, Rothstein L, et al. Radiosensitivity of human bone marrow granulocyte-macrophage progenitor cells and stromal colony-forming cells: effect of dose rate. Radiat Res 1986;107:205-15. [Crossref] [PubMed]
- Carmona R, Pritz J, Bydder M, et al. Fat composition changes in bone marrow during chemotherapy and radiation therapy. Int J Radiat Oncol Biol Phys 2014;90:155-63. [Crossref] [PubMed]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol 2002;30:513-28. [Crossref] [PubMed]
- Suryavanshi S, Sharma D, Checker R, et al. Amelioration of radiation-induced hematopoietic syndrome by an antioxidant chlorophyllin through increased stem cell activity and modulation of hematopoiesis. Free Radic Biol Med 2015;85:56-70. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Allegra CJ, Yothers G, O'Connell MJ, et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. J Natl Cancer Inst 2015;107:djv248. [Crossref] [PubMed]
- O'Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994;331:502-7. [Crossref] [PubMed]
- Scheithauer W, McKendrick J, Begbie S, et al. Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol 2003;14:1735-43. [Crossref] [PubMed]
- Engstrom PF, Arnoletti JP, Benson AB 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw 2009;7:778-831. [Crossref] [PubMed]