Eculizumab therapy for gemcitabine induced hemolytic uremic syndrome: case series and concise review
Introduction
The incidence of gemcitabine-induced hemolytic uremic syndrome (GiHUS) has been reported to be between 0.02% and 2.2% (1,2). A variety of therapies have been employed in the treatment of GiHUS with varying success. In some cases the discontinuation of drug will result in remission of HUS (3). The benefit of plasmapheresis in the treatment of atypical forms of HUS (aHUS) such as GiHUS has been questioned (4). Other treatment modalities have been used with varying rates of success including high dose corticosteroids, vincristine, and rituximab (3,5). Eculizumab is a monoclonal antibody directed against the complement protein C5 that has been recently approved for treatment of atypical HUS (3). We report four cases of GiHUS seen over 2-month period and successfully treated with eculizumab.
Patient 1
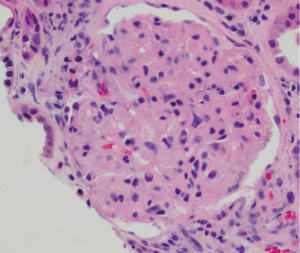
75 year-old male with stage IV squamous carcinoma of the lung was enrolled in a clinical trial (NCT01573780) with gemcitabine and TL-32711, a second mitochondrial-derived activator of caspase (SMAC) inhibitor after disease progression with his initial regimen. Baseline complete blood count (CBC) and serum chemistry panel were within normal limits (serum creatinine 1.03 mg/dL). He was noted to have hypertension and serum creatinine of 1.61 mg/d after six doses of gemcitabine (cumulative dose of 11,100 mg). Subsequently he was admitted for management of hypertensive urgency, and found to have hemoglobin (Hgb) of 8.8 g/dL and rising serum creatinine of 2.6 mg/dL. Urinalysis showed large amount of blood and 100 mg/dL protein. Platelet count was normal, lactic dehydrogenase (LDH) was 1,759 IU/L and haptoglobin <10 mg/dL. No schistocytes were seen in the peripheral blood smear (PBS). ADAMTS-13 activity was 74%. Complement (C3, C4) levels were normal. Renal biopsy was consistent with HUS (Figure 1) that was felt more likely to be caused by gemcitabine rather than the SMAC mimetic. After stopping the offending drug without improvement of renal function over 4 weeks, he received eculizumab 900 mg IV weekly for five doses followed by 1,200 mg IV every two weeks as maintenance (eight total doses). His last serum creatinine is 2.0 mg/dL. His LDH improved to 537 IU/dL, haptoglobin level was up to 42 mg/dL and Hgb to 11.6 g/dL.
Patient 2
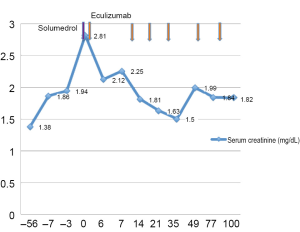
70 year-old male with metastatic pancreatic adenocarcinoma to the liver was enrolled in a clinical trial (NCT01125891) with gemcitabine and ON-0901910 but disease progression was noted in the liver. His baseline CBC and chemistry panel were within normal limits. His therapy was switched to gemcitabine and capecitabine on which he remained for 18 months. Capecitabine was discontinued due to palmar-plantar erythrodysesthesia but patient remained on gemcitabine. His serum creatinine steadily increased to 1.86 mg/dL after three cycles of single agent gemcitabine (after 53 total doses of gemcitabine with a cumulative dose of 99,540 mg) and platelet count dropped to 38×109/L. PBS showed rare schistocytes. LDH was 1,929 IU/L and haptoglobin <10 mg/dL. His C3 and C4 were within normal limits. Urinalysis showed large blood and 100 mg/dL protein. ADAMTS-13 activity was 79%. Gemcitabine was discontinued without improvement of CBC or creatinine for three weeks therefore, a kidney biopsy was done and showed thrombotic microangiopathy and acute interstitial nephritis. The patient received a dose of methylprednisolone 125 mg IV and eculizumab was started the next day. The patient received six doses of eculizumab resulting in favorable response in the renal function (creatinine 1.82 mg/dL) (Figure 2) and hemolysis markers (LDH 474 IU/L and haptoglobin 185 mg/dL).
Patient 3
73 year-old female with metastatic intrahepatic cholangiocarcinoma was treated with gemcitabine, capecitabine and bevacizumab per clinical trial (NCT01007552). After 24 cycles, bevacizumab was discontinued because of proteinuria and elevated serum creatinine (1.60 mg/dL). Serum creatinine rose to 1.95 mg/dL despite stopping bevacizumab. Laboratory investigation showed rare schistocytes on PBS, evidence of mild hemolysis and platelets of 143×109/L. Renal biopsy confirmed the diagnosis of thrombotic microangiopathy, and serum creatinine continued to rise up to 3.79 mg/dL even after fix weeks of stopping chemotherapy. Eculizumab alone (900 mg IV) was started weekly for four doses, followed by 1,200 mg IV as maintenance which she received for two doses. The patient had severe lower extremity edema and dyspnea with increasing serum serum creatinine for which received three hemodialysis treatments. Her serum creatinine then stabilized between 2.60-3.60 mg/dL but due to lack of viable options for further treatment she opted for supportive care and died four months later.
Patient 4
69 year-old female with stage IV squamous cell carcinoma of the lung with skeletal metastases was started on gemcitabine and carboplatin. During her fourth cycle she developed shortness of breath and lower extremity edema without rash, bleeding, fever or mental status changes. She was diagnosed with GiHUS based on a PBS with 6-8 schistocytes per high power field, anemia (Hgb 7.0 g/dL), thrombocytopenia (64×109/L), increased LDH (943 IU/L), low haptoglobin (<20 mg/dL), and increased creatinine (2.79 mg/dL from baseline 0.84 mg/dL). ADAMTS13 activity level was >95%. She was started on daily plasma exchange therapy (due to severe anemia and higher number of schistocytes noted on PBS) with partial improvement in her hematologic parameters but no improvement in her renal function. Plasma exchange was stopped and eculizumab was started at 900 mg weekly for 4 doses followed by one dose of 1,200 mg with normalization of hematologic parameters and improvement in renal function. The serum creatinine continued to improve to a level of 1.23 mg/dL at week 6 of treatment.
Discussion
Atypical HUS (aHUS) is a rare disease of uncontrolled complement activation associated with high mortality and progression to end stage renal disease (6). It may be caused by genetic mutations (7), ADAMTS-13 deficiency, HIV, malignancy, pregnancy, autoimmune diseases and drugs (8).
Typically, GiHUS is dose related with median cumulative dose reported to be 22 g/m2 (range, 4-81 g/m2) given over 7.5 months (range, 2-34 months) (9). In the four cases we report, the median cumulative gemcitabine dose was 21.2 g/m2. Other chemotherapeutic agents, have been implicated in causing thrombotic microangiopathy such as mitomycin C, cisplatin, carboplatin and bevacizumab (10,11). Of our four patients, two had received carboplatin, one had received cisplatin and one had received bevacizumab in past treatment regimens.
The pathophysiology of GiHUS is not well understood. In other forms of aHUS, it appears that there is uncontrolled proximal alternative pathway complement activation that leads to increased terminal membrane attack complex causing endothelial cell activation (6). There is evidence of activation of monocytes, neutrophils, and platelet activation and aggregation. Gemcitabine may directly damage endothelial cells, resulting in platelet aggregation and intravascular hemolysis.
The typical features of HUS may not always be seen in GiHUS. All patients were anemic but had varying degrees of thrombocytopenia. Rare or no schistocytes were identified on the peripheral smear of three individuals. The renal function changes occurred in a subacute fashion in all of these patients. Because of the lack of typical characteristics features of HUS, diagnosis was delayed. Three of four patients had subtle elevation of serum creatinine 2-6 months before suspecting HUS. Gemcitabine was stopped as it was suggested that 56% of these cases resolve with simply stopping the offending drug (12). Unfortunately, none of our patients showed improvement of renal function or hemolysis even after stopping gemcitabine for 3-5 weeks and eculizimab was initiated upon lack of clinically significant improvement even with other therapies such as corticosteroids. Eculizimab is a recombinant humanized monoclonal antibody that binds to complement C5 protein, inhibiting its cleavage, and thus preventing the generation of the terminal complement attack complex C5b-9 (13). It was initially approved for treating paroxysmal nocturnal hemoglobinuria in 2004 (14). It was first used in a case of atypical HUS in 2009 treated with a single dose leading to 8 months of stable renal function and without hemolysis (15). An international multicenter prospective phase II clinical trial (reported in abstract form) showed improvement of platelet counts (in 77% of the patients), improved renal function (in 88%) and avoidance of plasmapheresis or dialysis (in 88%) in a group of 17 adults. The most frequent adverse events were headache, anemia and diarrhea (3). Neisseria meningitidis vaccination is indicated at least two weeks prior to treatment (16).
In our experience treatment with eculizimab resulted in resolution of the microangiopathic hemolysis and thrombocytopenia in all four patients. Renal function improved significantly in all four patients but did not return to baseline. One patient required hemodialysis, but renal function subsequently improved. None of the patients had a severe adverse event related to eculizumab therapy.
In conclusion, GiHUS is a rare but serious entity with significant morbidity and mortality that requires early recognition and intervention. In view of the fact that these patients do not necessarily present with the typical finding of thrombocytopenia or schistocytes, our experience suggests that in patients treated with gemcitabine, rising creatinine maybe the only sign that should prompt testing for early detection of HUS. Eculizumab appears to be a well-tolerated, safe and effective treatment for GiHUS.
Acknowledgements
Disclosure: The authors of this manuscript have no financial support, funding or any conflict of interest to declare other than J.L who has a family member employed by Alexion Corporation.
References
- Fung MC, Storniolo AM, Nguyen B, et al. A review of hemolytic uremic syndrome in patients treated with gemcitabine therapy. Cancer 1999;85:2023-32. [PubMed]
- Desramé J, Duvic C, Bredin C, et al. Hemolytic uremic syndrome as a complication of gemcitabine treatment: report of six cases and review of the literature. Rev Med Interne 2005;26:179-88. [PubMed]
- American society of nephrology: renal week 2010: 43rd annual meeting & scientific exposition. P T 2011;36:48-9.
- Olie KH, Florquin S, Groothoff JW, et al. Atypical relapse of hemolytic uremic syndrome after transplantation. Pediatr Nephrol 2004;19:1173-6. [PubMed]
- Bharthuar A, Egloff L, Becker J, et al. Rituximab-based therapy for gemcitabine-induced hemolytic uremic syndrome in a patient with metastatic pancreatic adenocarcinoma: a case report. Cancer Chemother Pharmacol 2009;64:177-81. [PubMed]
- Ariceta G, Besbas N, Johnson S, et al. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol 2009;24:687-96. [PubMed]
- Caprioli J, Noris M, Brioschi S, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 2006;108:1267-79. [PubMed]
- Taylor CM, Machin S, Wigmore SJ, et al. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol 2010;148:37-47. [PubMed]
- Glezerman I, Kris MG, Miller V, et al. Gemcitabine nephrotoxicity and hemolytic uremic syndrome: report of 29 cases from a single institution. Clin Nephrol 2009;71:130-9. [PubMed]
- Casper ES, Green MR, Kelsen DP, et al. Phase II trial of gemcitabine (2,2'-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs 1994;12:29-34. [PubMed]
- Brodowicz T, Breiteneder S, Wiltschke C, et al. Gemcitabine-induced hemolytic uremic syndrome: a case report. J Natl Cancer Inst 1997;89:1895-6. [PubMed]
- Gore EM, Jones BS, Marques MB. Is therapeutic plasma exchange indicated for patients with gemcitabine-induced hemolytic uremic syndrome? J Clin Apher 2009;24:209-14. [PubMed]
- Schmidtko J, Peine S, El-Housseini Y, et al. Treatment of atypical hemolytic uremic syndrome and thrombotic microangiopathies: a focus on eculizumab. Am J Kidney Dis 2013;61:289-99. [PubMed]
- Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 2004;350:552-9. [PubMed]
- Nürnberger J, Philipp T, Witzke O, et al. Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med 2009;360:542-4. [PubMed]
- Westra D, Wetzels JF, Volokhina EB, et al. A new era in the diagnosis and treatment of atypical haemolytic uraemic syndrome. Neth J Med 2012;70:121-9. [PubMed]


