|
Original Article
Splenectomy ameliorates hematologic toxicity of hyperthermic intraperitoneal chemotherapy
Robert D Becher1, Perry Shen1, John H Stewart1, Greg Russell2, Joel F Bradley1, Edward A Levine1
1Surgical Oncology Service, Department of General Surgery, Wake Forest University School of Medicine, Winston-Salem, NC; 2Public Health Sciences, Wake Forest University School of Medicine, Winston-Salem, NC
Corresponding to: Edward A Levine, MD. Section of Surgical Oncology, Department of General Surgery, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157. Tel: 336-716-4276; Fax: 336-716-9758. Email: elevine@wfubmc.edu.
|
|
Abstract
Background: Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is a promising modality for peritoneal
carcinomatosis. Splenectomy is frequently required, however effect upon hematotoxicity is unknown.
Methods: 195 patients undergoing the procedure were evaluated and granulocyte colony stimulating factor administered
for white blood cell counts <4.0.
Results: 52% of 195 underwent splenectomy; average white blood cell and platelet nadirs were 6.1,172. Non-splenectomy
patients averaged white blood cell nadir 4.6, platelet nadir 164.1. Granulocyte colony stimulating factor administered
in 29% of splenectomy, 43% of non-splenectomy (P=0.043).
Conclusion: Splenectomy ameliorates hematotoxicity of hyperthermic intraperitoneal chemotherapy and significantly
reduces post-operative granulocyte colony stimulating factor requirements.
Key words
peritoneal carcinomatosis, hyperthermic intraperitoneal chemotherapy, splenectomy
J Gastrointest Oncol 2011; 2: 70-76. DOI: 10.3978/j.issn.2078-6891.2011.011
|
|
Introduction
Peritoneal dissemination or carcinomatosis is a terminal
disease and is one of the most common routes of spread
of abdominal carcinoma ( 1). Studies demonstrate that is
the primary cause of death in patients with resected intraabdominal
carcinomas ( 2-4). Cytoreductive surgery
alone has had limited utility in the treatment of peritoneal
carcinomatosis. Treatment of peritoneal carcinomatosis
with brachytherapy or external beam radiation therapy
has not been efficacious ( 5). Despite recent advances in
systemic chemotherapy, its effect is limited in part by the
plasma/peritoneal partition limiting entry of agents into the
peritoneum. Thus far, systemic chemotherapy has provided
modest improvement in survival for patients with peritoneal carcinomatosis ( 1, 6). Administration of intraperitoneal chemotherapy after
cytoreductive surgery delivers high and persistent local
concentrations of the chemotherapeutic agent, while
limiting systemic toxicity ( 7). Mild hyperthermia has been
shown to potentiate the effects of chemotherapeutic agents
such as cisplatin and mitomycin C, and these interactions
are enhanced under hypoxic conditions ( 8-10). Synergy
between hyperthermia and the chemotherapeutic agents
occurs independently of the cell cycle, which allows for
enhanced tumoricidal activity with brief exposures ( 11).
Mitomycin C is the most commonly administered agent
in hyperthermic intraperitoneal chemotherapy, however
oxaliplatin has been used as well. These agents are utilized
because of a highly favorable ratio between intraperitoneal
concentration versus plasma concentration over time
( 2, 12-14). The combination of cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy maximizes the
therapeutic benefit and has been shown to improve survival
and quality of life in select patients ( 7, 15, 16). The goal of cytoreduction is the resection of all gross
tumor, and this can necessitate resection of the peritoneum
with multivisceral resections, such as splenectomy. Current
morbidity rates range from 27% to 56% at centers which perform hyperthermic intraperitoneal chemotherapy
( 7), and one component of this is hematologic toxicity.
Splenectomy results in elevated postoperative cell counts,
primarily due to decreased clearance of senescent cells ( 17).
Therefore, we investigated the effect of splenectomy on
postoperative hematologic toxicity in a series of 195 patients
undergoing hyperthermic intraperitoneal chemotherapy.
|
|
Materials and methods
Approval for this retrospective study was obtained from the
Internal Review Board at Wake Forest University Medical
Center in Winston-Salem, North Carolina. We studied
a total of 195 patients with peritoneal carcinomatosis,
who underwent initial cytoreductive surgery followed
immediately by hyperthermic intraperitoneal
chemotherapy, between December 2003 and December
2007, at our tertiary care institution. All patients were
evaluated in the surgical oncology clinics preoperatively and
had pathologic confirmation of peritoneal carcinomatosis
prior to the procedure.
Cytoreductive surgery
Cy toreductive surger y was performed with the goal
of the removal of all gross tumor and involved organs,
peritoneum, or tissue deemed technically feasible and safe
for the patient. Any tumors adherent or invasive to vital
structures that could not be removed were cytoreduced
using the cavitational ultrasonic surgical aspirator (CUSA;
Valleylab, Boulder, Colo.). Peritonectomy procedures were
performed as indicated. The resection status of patients
was judged after cytoreductive surgery using the following
classification: R0-complete removal of all visible tumor and
negative cytologic findings or microscopic margins; R1-
complete removal of all visible tumor and positive postperfusion
cytologic findings or microscopic margins; R2aminimal
residual tumor, nodule(s) measuring 0.5 cm or
less; R2b-gross residual tumor, nodule greater than 0.5 cm
but less than or equal to 2 cm; and R2c-extensive disease
remaining, nodules greater than 2 cm. Splenectomy was
performed when gross disease was found on the capsule
of the spleen, indicating a higher burden of peritoneal
dissemination requiring more extensive surgery.
Hyperthermic intraperitoneal chemotherapy
Patients were passively cooled to a core temperature of
approximately 34oC to 35oC by passive measure (i.e.,
not warming airway gases or intravenous solutions and
cooling the room). After cytoreductive surgery was
completed, peritoneal perfusion inf low and outf low
catheters were placed percutaneously into the abdominal cavity. Temperature probes were placed on the inflow and
outflow catheters. The abdominal skin incision was closed
temporarily with a running cutaneous suture to prevent
leakage of peritoneal perfusate. A perfusion circuit was
established with approximately 3 L of Ringer’s lactate.
Flow rates of approximately 800 to 1000 mL/min were
maintained using a roller pump managed by the pump
technician. The circuit continued through a pump, then a
heat exchanger and then back to the patient.
Constant temperature monitoring was performed at all
temperature probes. Once inf low temperature exceeded
38.5 oC, 30 mg of mitomycin C was added to the perfusate.
At 60 minutes an additional 10 mg of mitomycin C was
added to keep mitomycin C perfusate concentrations
higher than 5μg/mL. A maximum inf low temperature
of 42.0 oC was realized during perfusion, with a target
outf low temperature at the pelvis of 40 oC. The abdomen
was gently massaged throughout perfusion to improve
drug distribution to all peritoneal surfaces. Total planned
perfusion time after the initial addition of mitomycin C was
120 minutes. In certain patients (elderly individuals, those
with extensive previous chemotherapy, those with inanition
or poor performance status, and patients having extensive
peritoneal stripping during surgery), reductions in the dose
of mitomycin C (to 30 mg total) or perfusion time (to 60-90
minutes) were made due to concerns about potential toxic
effects. Oxaliplatin was administered to a total of 21 of the
195 patients (11%) at a dose of 200 mg/m 2 for a total of 120
minutes; no similar reductions in dosage were needed for
oxaliplatin patients ( 18). Postoperatively, patients had complete blood counts
determined daily until discharge. Treatment wit h
recombinant granulocy tecolony stimulating factor
(Neupogen) at a dose of 5μg/kg/day was initiated when their
white blood cell counts were <4,000/mm. The granulocyte
colony stimulating factor was continued until the white
blood cell was >10,000/mm, a value in the normal range for
our laboratory ( 19). Hematologic toxicity was graded on a
standard scale from 0-5, with 5 being most severe using the
National Cancer Institute’s Common Terminology Criteria
for Adverse Events standard criteria ( 20).
|
|
Results
One hundred ninety five patients (101 women, 94
men), aged 25 to 81 years (mean 53), with peritoneal
carcinomatosis underwent cytoreductive surgery with
hyperthermic intraperitoneal chemotherapy. The primary
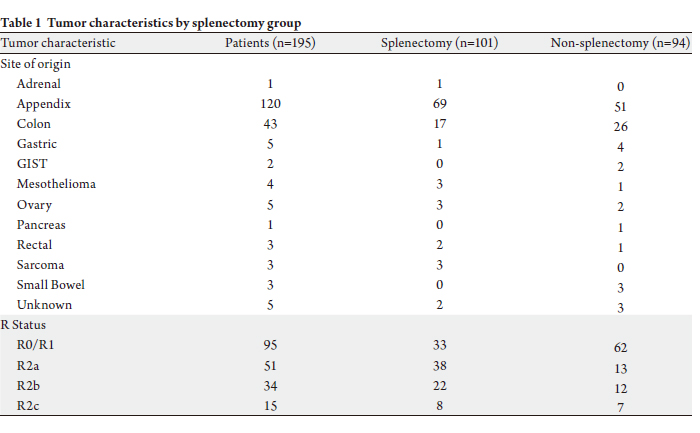
site of origin of the peritoneal carcinomatosis and R
resection status are shown in Table 1. There were 101
patients (52%) who underwent a splenectomy during cytoreductive surgery. Splenectomy rates were significantly
different by R resection status (P<0.0001), with 33
splenectomies in the 95 R0/R1 resections (35%), 38
splenectomies in the 51 R2a resections (75%), 22 in the 34
R2b resections (65%), and 8 in the 15 R2c resections (53%).
Overall, 6 of 101 patients (6%) in the splenectomy group
and 3 of 94 patients (3%) in the non-splenectomy group died
within 30 days of cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy (not statistically different).
Cytopenia contributed to death from sepsis in 4 patients
(4%) in the splenectomy group and 1 patient (1%) in the
non-splenectomy group (not statistically different).
The average hospital stay was significantly different
(P=0.001) between the two groups, with the splenectomy
group average being 20 days (median 11 days) and the
average stay for the non-splenectomy group being 12 days
(median 9 days).
Dose reduction of mitomycin C was required in 2
patients in the non-splenectomy group and 2 patients in the
splenectomy group. For patients in the splenectomy group,
the average white blood cell nadir was 6.1 +/- 3.4 (range 0.3
to 14.7) on day 7.2. The average absolute neutrophil count
was 5.2 +/- 3.6 (range 0.1 to 13.4), the average platelet nadir
was 172.0 +/- 81.9 (range 3.0 to 381.0), and the average
hemoglobin nadir was 7.5 +/- 1.0 (range 4.9 to 10.3). For
patients in the non-splenectomy group, the average white
blood cell nadir was 4.6 +/- 2.4 (range 0.5 to 13.2) on day 6.0. The average absolute neutrophil count was 3.9 +/- 2.7
(range 0.2 to 14.5), the average platelet nadir was 164.1 +/-
73.0 (range 6.0 to 426.0), and the average hemoglobin nadir
was 8.2 +/- 1.8 (range 4.4 to 13.9).
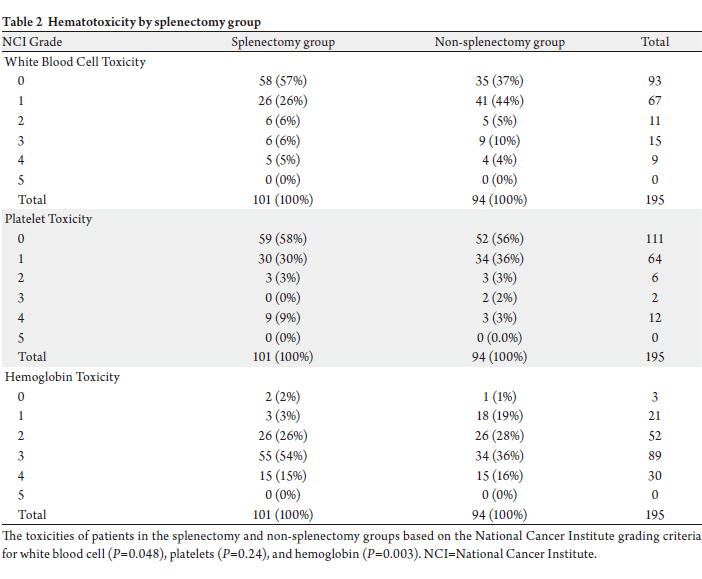
Hematologic toxicity grade by National Cancer
Institute criteria is shown for white blood cell, platelets,
and hemoglobin in Table 2. White blood cell toxicity was
significantly lower in the splenectomy group compared to
the non-splenectomy group (P=0.048). Platelet toxicity
was not statistically significantly different between the two
groups (P=0.24). Hemoglobin toxicity was significantly
worse in the splenectomy group (P=0.003). There was no
statistically significant difference in hematologic toxicity
between those receiving mytomycin C and those receiving
oxaliplatin (P=0.754).
Granulocyte colony stimulating factor was administered
in 29% of splenectomy patients versus 43% of nonsplenectomy
patients (P=0.043). Granulocyte colony
stimulating factor was administered for an average of 2.9 +/-
2.1 days (range 1.0 to 8.0) in the splenectomy group, versus
an average of 3.3 +/- 3.3 days (range 1.0 to 18.0) in the nonsplenectomy
group. The difference in the average number of
days treated with granulocyte colony stimulating factor was
not statistically significant (P=0.61).
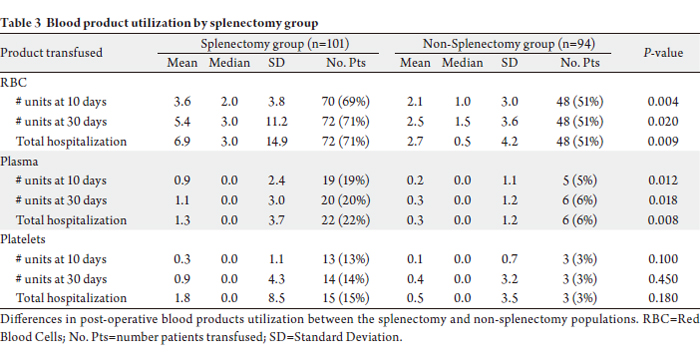
During the post-operative period, there were significant
differences in the number of red blood cell transfusions
required for the splenectomy group compared to the non-splenectomy group (Table 3). Over the first 10 postoperative
days, there was an average of 3.6 +/- 3.8 red
blood cell transfusions (median 2.0; range 0.0 to 19.0) in
patients in the splenectomy group versus 2.1 +/- 3.0 red
blood cell transfusions (median 1.0; range 0.0 to 13.0)
in the non-splenectomy group (P=0.004). A total of 70
(69%) splenectomy patients and 48 (51%) non-splenectomy
patients got red blood cell transfusions over the first 10
days. Over the entire hospitalization, there was an average
of 6.9 +/- 14.9 red blood cell transfusions (median 3.0;
range 0.0 to 123.0) in patients in the splenectomy group
versus 2.7 +/- 4.2 red blood cell transfusions (median 0.5;
range 0.0 to 25.0) in the non-splenectomy group (P=0.009).
A total of 72 (71%) splenectomy patients and 48 (51%) nonsplenectomy
patients got red blood cell transfusions over
the entire hospitalization.
The difference in plasma transfusions post-operatively (Table 3). Over the first 10 post-operative days, there was
an average of 0.9 +/- 2.4 plasma transfusions (median 0.0;
range 0.0 to 13.0) in patients in the splenectomy group
versus 0.2 +/- 1.1 platelet transfusions (median 0.0; range
0.0 to 6.0) in the non-splenectomy group (P=0.012). A
total of 19 (19%) splenectomy patients and 5 (5%) nonsplenectomy
patients got plasma transfusions over the
first ten days. Over the entire hospitalization, there was an
average of 1.3 +/- 3.7 transfusions (median 0.0; range 0.0
to 27.0) in patients in the splenectomy group versus 0.3 +/-
1.2 platelet transfusions (median 0.0; range 0.0 to 7.0) in
the non-splenectomy group (P=0.008). A total of 22 (22%)
splenectomy patients and 6 (6%) non-splenectomy patients
got plasma transfusions over the entire hospitalization.
There was no significant difference in the number of
platelet transfusions between the splenectomy and nonsplenectomy
groups at 10 days post-operatively (P=0.10),
30 days post-operatively (P=0.45), or during the total hospitalization (P=0.18) (Table 3). The difference in
cryoprecipitate transfusions was not significant.
|
|
Discussion
Utilizing cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy together is a promising
modality for the treatment of patients with a variety
of peritoneal surface malignancies . However, the
morbidity and mortality of hyperthermic intraperitoneal
chemotherapy are significant, principally due to the extent
of surgery necessary for optimal cytoreduction ( 21). The
rates of morbidity range from 27 to 56% at various centers
that perform hyperthermic intraperitoneal chemotherapy,
and are thought to be related to the extent of carcinomatosis,
duration of the operation, preoperative performance status
of the patient, and the number of anastomoses ( 7, 22). The
most common complications are abscess, fistula, prolonged
ileus, pneumonia and hematologic toxicity ( 7, 23). Splenectomy is well known to result in postoperative
leukocytosis and thrombocytosis ( 17, 24); this increase in
the white blood cell and platelet counts have previously
been shown to occur after cytoreductive surgery
without hyperthermic intraperitoneal chemotherapy for
carcinomatosis ( 17). In patients undergoing cytoreductive
surgery together with hyperthermic intraperitoneal
chemotherapy, only one previous study which we are aware
of assessed the relationship between splenectomy and
postoperative neutropenia; no association was found ( 25).
Therefore, we chose to examine the effect of splenectomy on hematologic toxicity after hyperthermic intraperitoneal
chemotherapy with cytoreductive surgery, and assess the
use of granulocyte colony stimulating factor. In the patients who underwent splenectomy, the
white cell nadir was higher, and therefore, splenectomy
ameliorated the neutropenia attendant to hyperthermic
intraperitoneal chemotherapy. This resulted in a significant
decrease in the need for recombinant granulocyte colony
stimulating factor support using a standard protocol
for its utilization. The platelet nadir was also higher in
the splenectomy group, though this did not result in a
significant difference in platelet utilization.
Since patients who underwent splenectomy in this
experience had disease seen grossly on the organs,
splenectomy also correlates with increased tumor burden.
Consequently, it is not surprising that a significantly
higher grade hemoglobin toxicity was seen in the
splenectomy cohort as they required a more extensive
operative intervention. This is consistent with the lower
hemoglobin nadir in the splenectomy group, and translated
into significantly more red blood cell transfusions in this
population.
Furthermore, given the increased peritoneal
dissemination in splenectomy patients compared to nonsplenectomy
patients, and thus the need for more extensive
multivisceral resection, it is also not surprising that the
splenectomy cohort had, on average, a significantly longer
hospital stay. Additionally, while a higher proportion of
splenectomy patients expired, there was not a statistically
significant difference in the mortality between the two groups or in the proportion of patients who expired from
cytopenia.
Splenectomy is associated with morbidities
including atelectasis, pleural effusion, pancreatic injury,
thrombocy tosis, subphrenic abscess, and pancreatic
pseudocyst formation ( 26). A feared complication after
splenectomy is overwhelming sepsis, which has an overall
mortality of 50%, and may occur between 24 days to 65
days after surgery ( 27). Pneumococcus is the causative
organism in over 60% of cases. Our current standard of
care involves vaccination with polyvalent pneumococcal
vaccine, H. influenzae type b conjugate, and meningococcal
polysaccharide vaccine within 2 weeks of splenectomy
( 28). We routinely administer, and suggest vaccinations for
patients undergoing splenectomy. When splenectomy can be
anticipated based upon imaging, preoperative vaccination
is preferred. Utilizing this vaccination protocol, we have not
encountered a case of overwhelming post-splenectomy sepsis
in this patient group to date. We acknowledge that our study has limitations. First,
our conclusions are drawn from a limited sample size of
195 patients. Concomitantly, in addition to the specific
differences between the splenectomy and non-splenectomy
patient populations described, other factors may have
contributed to our conclusions. Furthermore, due to the
low number of patients receiving only oxaliplatin (n=21) we
caution making definitive conclusions from a subanalysis of
patients receiving only mytomycin C and only oxaliplatin.
Lastly, this study is a retrospective analysis, and therefore is
prone to the potential limitations and biases therein.
|
|
Conclusion
Splenectomy ameliorates the hematologic toxicity attendant
to hyperthermic intraperitoneal chemotherapy. Further, it
significantly reduces the number of patients who require
post-operative growth factor support. To our knowledge,
this is the first report of this finding. While we do not suggest
routine splenectomy as part of cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy, this effect of
amelioration of hematologic toxicity should be considered
when contemplating splenectomy during cytoreductive
procedures prior to chemoperfusion.
|
|
References
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J,
et al. Peritoneal carcinomatosis from non-gynecologic malignancies:
results of the EVOCAPE 1 multicentric prospective study. Cancer
2000;88:358-63.[LinkOut]
- Sugarbaker PH, Cunliffe WJ, Belliveau J, de Bruijn EA, Graves T, Mullins RE, et al. Rationale for integrating early postoperative intraperitoneal
chemotherapy into the surgical treatment of gastrointestinal cancer.
Semin Oncol 1989;s16:83-97.[LinkOut]
- Loggie BW, Fleming RA, McQuellon RP, Russell GB, Geisinger KR,
Levine EA. Prospective trial for the treatment of malignant peritoneal
mesothelioma. Am Surg 2001;67:999-1003.[LinkOut]
- Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from
colorectal cancer. Br J Surg 2002;89:1545-50.[LinkOut]
- Wong CS, Harwood AR, Cummings BJ, Keane TJ, Thomas GM, Rider
WD. Total abdominal irradiation for cancer of the colon. Radiother
Oncol 1984;2:209-14.[LinkOut]
- Dedrick RL. Theoretical and experimental bases of intraperitoneal
chemotherapy. Semin Oncol 1985;s12:1-6.[LinkOut]
- Stewart JH, Shen P, Levine EA. Intraperitoneal hyperthermic
chemotherapy for peritoneal surface malignancy: current status and
future directions. Ann Surg Oncol 2005;12:765-77.[LinkOut]
- Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, et al.
Intraperitoneal chemohyperthermia using a closed abdominal procedure
and cytoreductive surgery for the treatment of peritoneal carcinomatosis:
morbidity and mortality analysis of 216 consecutive procedures. Ann
Surg Oncol 2003;10:863-9.[LinkOut]
- Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, El OA, et
al. Heated intra-operative intraperitoneal oxaliplatin after complete
resection of peritoneal carcinomatosis: pharmacokinetics and tissue
distribution. Ann Oncol 2002;13:267-72.[LinkOut]
- Teicher BA, Kowal CD, Kennedy KA, Sartorelli AC. Enhancement by
hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic
tumor cells. Cancer Res 1981;41:1096-9.[LinkOut]
- Barlogie B, Corry PM, Drewinko B. In vitro thermochemotherapy of
human colon cancer cells with cis-dichlorodiammineplatinum (II) and
mitomycin C. Cancer Res 1980;40:1165-8.[LinkOut]
- Sugarbaker PH, Graves T, DeBruijn EA, Cunliffe WJ, Mullins RE,
Hull WE, et al. Early postoperative intraperitoneal chemotherapy
as an adjuvant therapy to surger y for peritoneal carcinomatosis
from gastrointestinal cancer: pharmacological studies. Cancer Res
1990;50:5790-4.[LinkOut]
- Fernández-Trigo V, Stuart OA, Stephens AD, Hoover LD, Sugarbaker
PH. Surgically directed chemotherapy: heated intraperitoneal lavage
with mitomycin C. Cancer Treat Res 1996;81:51-61.[LinkOut]
- Sweitzer KL, Nathanson SD, Nelson LT, Zachary C. Irrigation does not
dislodge or destroy tumor cells adherent to the tumor bed. J Surg Oncol
1993;53:184-90.[LinkOut]
- Piso P, Bektas H, Werner U, Schlitt HJ, Kubicka S, Bornscheuer
A, et al. Improved prognosis following peritonectomy procedures
and hyperthermic intraperitoneal chemotherapy for peritoneal
carcinomatosis from appendiceal carcinoma . Eur J Surg Oncol
2001;27:286-90.[LinkOut]
- McQuel lon RP, Logg ie BW, Fleming R A, Russel l GB, Lehma n
AB, Rambo TD. Quality of life after intraperitoneal hyperthermic
chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol
2001;27:65-73.[LinkOut]
- Bidus MA, Krivak TC, Howard R, Rose GS, Cosin J, Dainty L, et al.
Hematologic changes after splenectomy for cytoreduction: implications
for predicting infection and effects on chemotherapy. Int J Gynecol
Cancer 2006;16:1957-62.[LinkOut]
- Stewart JH 4th, Shen P, Russell G, Fenstermaker J, McWilliams L,
Coldrun FM, et al. A phase I trial of oxaliplatin for intraperitoneal
hyperthermic chemoperfusion for the treatment of peritoneal surface
dissemination from colorectal and appendiceal cancers. Ann Surg
Oncol 2008;15:2137-45.[LinkOut]
- NCCN [Internet]. NCCN clinicalpractice guidelines in oncology:
myeloid growth factors. c2010-[cited 2010 Aug 16]. Available from:
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.[LinkOut]
- National Cancer Institute’s Cancer Therapy Evaluation Program.
Common terminology criteria for adverse events (CTCAE), version
3.0. NIH Publication No. 03-5410. DCTD, NCI, NIH, DHHS March
31, 2003 (http://ctep.cancer.gov); 2003.[LinkOut]
- Ahmad SA, Kim J, Sussman JJ, Soldano DA, Pennington LJ, James
LE, et al. Reduced morbidity following cytoreductive surgery and
intraperitoneal hyperthermic chemoperfusion. Ann Surg Oncol
2004;11:387-92.[LinkOut]
- Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag
G, et al. Morbidity and mortality analysis of 200 treatments with
cytoreductive surgery and hyperthermic intraoperative intraperitoneal
chemotherapy using the coliseum technique. Ann Surg Oncol
1999;6:790-6.[LinkOut]
- Levine EA, Stewart JH 4th, Russell GB, Geisinger KR, Loggie BL,
Shen P. Cytoreductive surgery and intraperitoneal hyperthermic
chemotherapy for peritoneal surface malignancy: experience with 501
procedures. J Am Coll Surg 2007;204:943-53; discussion 953-5.[LinkOut]
- Weng J, Brown CV, Rhee P, Salim A, Chan L, Demetriades D, et al.
White blood cell and platelet counts can be used to differentiate
between infection and the normal response after splenectomy for
trauma: prospective validation. J Trauma 2005;59:1076-80.[LinkOut]
- Lambert LA, Armstrong TS, Lee JJ, Liu S, Katz MH, Eng C, et
al. Incidence, risk factors, and impact of severe neutropenia after
hyperthermic intraperitoneal mitomycin C. Ann Surg Oncol
2009;16:2181-7.[LinkOut]
- Pate JW, Peters TG, Andrews CR. Postsplenectomy complications. Am
Surg 1985;51:437-41.[LinkOut]
- Waghorn DJ. Overwhelming infection in asplenic patients: current
best practice preventive measures are not being followed. J Clin Pathol
2001;54:214-8.[LinkOut]
- Shatz DV, Schinsky MF, Pais LB, Romero-Steiner S, Kirton OC,
Carlone GM. Immune responses of splenectomized trauma patients to
the 23-valent pneumococcal polysaccharide vaccine at 1 versus 7 versus
14 days after splenectomy. J Trauma 1998;44:760-5; discussion 765-6.[LinkOut]
Cite this article as:
Becher R, Shen P, Stewart J, Russell G, Bradley J, Levin E. Splenectomy ameliorates hematologic toxicity of hyperthermic intraperitoneal chemotherapy. J Gastrointest Oncol. 2011;2(2):70-76. DOI:10.3978/j.issn.2078-6891.2011.011
|





