Epidemiological profile of gastric cancer in the northwestern region of Algeria: about 116 cases
Introduction
Gastric cancer (GC) remains a public health issue despite the global decrease in incidence over the last decades. Approximately one million of new cases were diagnosed (6.8% of all cancers) making this digestive neoplasia the fifth most common malignancy and the third leading cause of cancer related-death in both genders worldwide (8.8% of the total). The risk is particularly higher in Eastern Asia were half of the world total occurs mainly in China and Japan. Although the African continent is considered as a low incidence area, 13,216 new cases of stomach cancer were diagnosed and 12,000 cases of death were recorded during 2012 (1).
Most common histologic entity of GC is adenocarcinoma accounting for 90% of all stomach tumors (2). Sixty percent to Seventy percent of GCs represent the final step of mucosal precancerous inflammatory process induced by Helicobacter pylori (HP) infection. The pre-neoplastic cascade is initiated by atrophic chronic gastritis (ACG) which may persist and advance to intestinal metaplasia (IM) than dysplasia. ACG and IM are considered as reversible steps regressing by the bacterium eradication (3,4).
Aiming to reveal the profile of GC in the Northwestern region of Algeria, the current study, carried out at the level of the University Hospital Abdelkader Hassani, describes the epidemiological, clinical and histopathological aspects of stomach cancer and determines the gastric precancerous lesions among cancerous patients admitted at the level of the Gastroenterology Department.
Methods
We retrospectively analyzed the patients’ medical records admitted at the gastroenterology department between 2010 and 2015. One hundred and sixteen patients with histologically confirmed GC who have not received an eradication treatment of HP were included. Patients with benign gastric lesions or with non-proven malignant lesions were excluded. The whole studied population received physical examination, upper gastrointestinal endoscopy combined to biopsy and abdominal and pelvic echography.
Biopsy specimens were performed during endoscopy and fixed in 10% formalin before being dehydrated then embedded in paraffin blocks at the level of the pathology department for routine examination. The paraffin blocks were censored by a rotating microtome then fixed on slides before being stained with hematoxylin and eosin (H & E). The interpretation of the slides was made at low and high magnifications using an optical microscope.
Statistical analysis
Enrolled data (age, gender, clinical and histopathological records) were computed and analyzed using Microsoft Excel 2013 and IBM SPSS Statistics 20 (Statistical Package for the Social Sciences, IBM Corporation; Chicago, IL, USA. August 2011). The mean and standard deviations were calculated for quantitative variables, Student t-test and Chi squared test were executed to compare categorical variables. Significance level was accepted at P value of 0.05 or less.
Ethical considerations
The study was approved by the scientific and ethical committee of author’s institution.
Ethical consent was acquired from the departments’ chiefs where the study was conducted with a complete confidentiality of the patients’ data sustained during the whole work.
Results
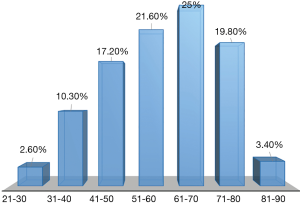
The mean age of the whole studied cohort was 58.96±14.75 years with 60.03±13.69 years in male vs. 57.49±16.11 years in female. The age distribution varied from 21 to 90. GC was more recurrent in the age group of 61–70 years followed by 51–60 years with a rate of 25% and 21.55% respectively as shown on Figure 1.
Masculine predominance was revealed with a sex ratio of 1.36; 57.76% of all cases were diagnosed in male and 42.24% in female.
Clinically, epigastralgia revealed the presence of the neoplasia in 57.8% of cases, followed by vomiting and gastrointestinal bleeding i.e., up or low bleeding, which had occurred in 17.2% and 12.1%, respectively. 6.9% and 6% of patients presented ascites and dysphagia, correspondingly.
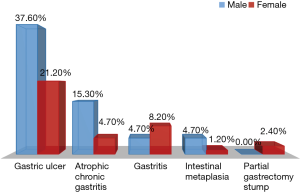
In the current study, precancerous lesions were found in 73.24% of cases: we counted 50 cases of gastric ulcer (58.8%), 17 cases of ACG (20%) and 11 cases of gastritis i.e., acute or chronic gastritis (12.9%). Reduced rates were recorded for IM (5.9%) and partial gastrectomy stump (2.4%) as shown on Figure 2. No significant differences were observed between both genders according to the cohort distribution by gender and revealing signs and by gender and gastric precancerous lesions with P=0.748 and P=0.071, respectively.
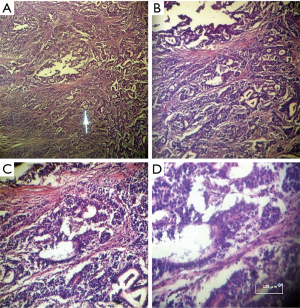
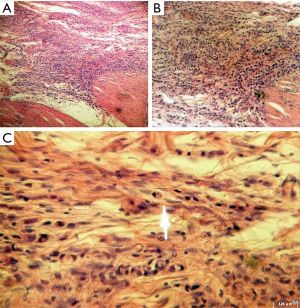
Histologic proof showed that 72% of cases were adenocarcinoma as illustrated on Figure 3, 11.2% were MALT lymphoma, 9.3% were linitis plastica (Figure 4) and 7.5% were gastrointestinal stromal tumors (GIST). The association between gender and tumor histology has shown a statistical significance with a P value of 0.01: adenocarcinoma was expressively higher among male patients and linitis plastica was significantly related to female gender (Table 1) and was typically diagnosed in women aged between 41 and 50 years.
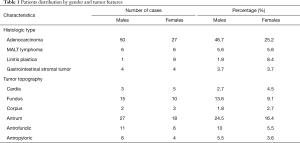
Full table
The tumor topography was presented mainly in the antral and fundic regions accounting for 40.9% and 22.7% respectively. 15.5% of tumors were situated at the level of the antropyloric region and 9.1% of them were of an antrofundic site. GC was located at the level of the cardia in 7.3% of cases, and at the level of the corpus in 4.5% (Table 1). No statistical significance existed between the two genders according to the tumor topography (P=0.778).
Discussion
Stomach cancer represents the ninth malignancy diagnosed and the third digestive neoplasia detected in African continent according to GLOBOCAN 2012. The occurrence of GC in the age group of 30–40 years is rare. However, its incidence increases with age especially among individuals aged over 60 years (1).
The mean age reported in our study agrees with data observed in a Moroccan study conducted by Mellouki et al., who stated a mean age of 58±13.4 years (5). However, the mean age recorded in France and South Korea was higher; 72.7±11 and 59.2±11.9 years, respectively (6,7). In our context, the occurrence of GC among young patients especially those of the age group of 51–60 years could be related to HP infection and the non-management of precancerous lesions, essentially gastric ulcer and gastro-epithelial atrophy.
We should underline that several studies reported a masculine predominance supporting our findings (8-10).
The reason of GC recurrence among male patients is possibly related to the host genetic and physiological profile and their high exposure to risk factors compared to female gender.
Our clinical data are similar to those reported in a series of research; epigastralgia was in the forefront of clinical signs with a rate of 75% in a Malian population and 94% in Togo (11,12). We found a vomiting frequency higher than Bouglouga et al. and lesser than Dembélé et al. (9,13). The rate of gastrointestinal bleeding was lower than Mellouki et al. [2014] (5).
Those signs were not specific to GC, which leads to say that their presence is not necessarily a proof of the existence of gastric neoplasia.
Rates of gastric precancerous lesions reported in our study do not concord with some research carried out in Africa. Bouglouga et al. recorded a frequency of gastric ulcer of 25% and counted a unique case of chronic gastritis. According to Sacko et al. the rate of gastric ulcer was even more reduced (3.57%). However, the frequency of partial gastrectomy stump was close to our results (1.1%) (9,11).
Correa & Piazuelo established the relationship between precancerous lesions and GC: ACG induced by HP infection progresses towards IM leading to the development of a GC. This cascade is revocable in anticipation of the stratum IM, which has prevented the development of cancer by the eradication of HP (4). According to Kuipers, atrophy was found in 97% of patients with GC and the frequency of IM varied from 45–98% depending on the cancer subtype i.e., 45% for the diffuse subtype and 98% for the intestinal subtype (14).
No available data have been reported about the prevalence of HP infection in Algerian population or in cancerous cohorts. In the current study, the detection of HP was performed in limited cases, which prevented us from exploiting this risk factor and from correlating it to the studied variables.
However, the fact that Algeria is a developing country, lead us to emphasize on the lack of healthcare institutions as well as the low socioeconomic level of citizens and their poor awareness about the necessity of medical investigations in case of emergence of any symptoms related to the digestive tract. All these factors could promote the spread of infectious diseases witch may persist and advance to malignancies.
In the current study, the representable frequency of gastric ulcer (58.8%) was due to the aggressive symptomatology associated to this mucosal lesion. Usually, patients refer to their physicians to relieve those symptoms. However, gastric ulcer could not be totally treated after the absence of the symptoms; the good and complete management of this lesion depends on the competence of physicians and their diagnosis additionally to the patients’ attitude and their health status at the moment of the diagnosis. Concerning ACG and IM, those lesions are asymptomatic ones and of late diagnosis, which explain the low rates registered in our study.
The majority of GC studies reported that adenocarcinoma was the most represented histological type with a frequency exceeding 90% confirming our results (15-17). We found a higher rate of malignant lymphoma than in Cameroon (9.5%), and a reduced rate of linitis plastica compared to the same study (26.2%) (18), and a frequency of GIST advanced than that stated by Dembélé et al. (1.6%) (13).
In our context, linitis plastica was more recurrent among female patients especially in those aged between 41 and 50 years. This rare subtype of gastric adenocarcinoma is related to genetic mutation of CDH1 (E-cadherin gene) encoding the intracellular adhesion protein E-cadherin, in this pattern the loss of expression of this protein explains its diffuse appearance (19). Menopausal female may be more susceptible to develop this GC subtype due to their physiological profile essentially the hormonal one: hormonal disorders could promote the occurrence of neoplasia including gastric signet ring cell carcinoma.
Some African authors reported in their studies that the antropyloric site was encountered in 52.3% and 74.11% of the patients respectively contrasting our results where the frequency of this location was reduced (9.1%) (8,17). According to Daouda et al., 79% gastric tumors were located at the antral and cardiac level, confirming our results concerning the antral site (40.9%) and opposing those related to the cardiac location (7.3%) (20). We noticed that the cardiac location was evoked in 7.3% of the studied population. Those results were close to those found in South Korea (4–5%) and did not approve those found in Cameroon (30.4%) (18,21).
The antral locations (antral and antropyloric) are merely recurrent due to the existence of the bacterium HP at this level. This Class I human carcinogen plays a key role in the tumorigenesis process by inducing an inflammatory cascade leading to the development of gastric neoplasia.
Conclusions
Stomach cancer remains one of the morbid cancers and represents a global public health issue, especially in Algeria. In our context, gastric ulcer represented the main precancerous lesion detected among the studied cohort, adenocarcinoma was the histologic type recurrently diagnosed and was significantly specific to male gender. The majority of tumors were located at the level of the antral region. In Algerian populations, more data about the prevalence of HP infection are still needed to reveal the biologic status and behavior of this malignancy, essentially among young adults. In order to prevent the occurrence of GC, a better management of gastric precancerous lesions, using the eradication of HP in the first-degree relatives of an individual with GC, may considerably reduce the risk of its development. Nevertheless, more efforts are still required to improve the early detection of this disease and to reduce the diagnostic delay by making paraclinical examinations, in this case upper digestive endoscopy, more accessible.
Acknowledgements
We would like to thank the Gastroenterology Department and the Pathology Department of University Hospital Hassanni Abdelkader of Sidi-Bel-Abbes especially Dr. Fatima Zohra Tayebi assistant in Pathology and Dr. Mohamed Ziani assistant in Gastroenterology for their guidance and insight during the realization of this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the scientific and ethical committee of Djillali Liabes University (D.E 04-180-2005).
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012. Cancer Incidence and Mortality Worldwide: IARC CancerBase 2013. Available online: http://globocan.iarc.fr
- Carl-McGrath S, Ebert M, Röcken C. Gastric adenocarcinoma: epidemiology, pathology and pathogenesis. Cancer Therapy 2008;6:877-93.
- Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther 2014;40:250-60. [Crossref] [PubMed]
- Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis 2012;13:2-9. [Crossref] [PubMed]
- Mellouki I. Epidémiologie du cancer gastrique: expérience d’un centre hospitalier marocain. Pan Afr Med J 2014;17:42. [Crossref] [PubMed]
- Jeong O, Park YK. Clinicopathological Features and Surgical Treatment of Gastric Cancer in South Korea: The Results of 2009 Nationwide Survey on Surgically Treated Gastric Cancer Patients. J Gastric Cancer 2011;11:69-77. [Crossref] [PubMed]
- Jézéquel J, Bessaguet C, Verveur C, et al. Trends in incidence, management, and survival of gastric and cardia carcinomas in the area of Finistere (France) between 1984 and 2003. Eur J Gastroenterol Hepatol 2010;22:1412-9. [PubMed]
- Fadlouallah M, Krami H, Errabih I, et al. Le cancer gastrique : aspects épidémiologiques au Maroc. African Journal of Cancer 2015;7:8-15. [Crossref]
- Bouglouga O, Lawson-Ananissoh LM, Bagny A, et al. Stomach cancer: Epidemiological, clinical and histological aspects at the Lome Campus teaching hospital (Togo). Med Santé Trop 2015;25:65-8. [PubMed]
- Dikshit RP, Mathur G, Mhatre S, et al. Epidemiological review of gastric cancer in India. Indian J Med Paediatr Oncol 2011;32:3-11. [Crossref] [PubMed]
- Sacko O, Soumare L, Camara A, et al. Prise en charge des tumeurs malignes gastriques dans le service de chirurgie « A » du CHU du point G à propos de 84 cas. Mali Medical 2014;29:49-52.
- Diarra M, Konate A, Traoré CB, et al. Epidémiologie des cancers digestifs en milieu hospitalier à Bamako. HEGEL - HEpato-GastroEntérologie Libérale 2012;2:12-22.
- Dembélé BT, Togo A, Kanté L, et al. Cancer gastrique non résécable dans le service de chirurgie générale CHU Gabrièle Touré Bamako. Mali Medical 2012;27:14-8.
- Kuipers EJ. Review article: relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther 1998;12:25-36. [Crossref] [PubMed]
- Diarra MT, Konate A, Diarra AN, et al. Les caractéristiques épidémiologiques et pronostiques du cancer de l’estomac en milieu urbain au Mali. Mali Medical 2014;29:45-8.
- Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700-13. [Crossref] [PubMed]
- Effi AB, N’Dah KJ, Doukouré B, et al. Profil histopathologique des cancers digestifs primitifs en Côte-d’Ivoire. Journal Africain d’Hépato-Gastroentérologie 2011;5:93-8. [Crossref]
- Ankouane F, Kowo M, Nonga B, et al. Histological Types of Gastric Cancer and Helicobacter pylori Infection in Yaoundé. Journal of Cancer Therapy 2015;6:701-8. [Crossref]
- Carcas LP. Gastric cancer review. J Carcinog 2014;13:14. [Crossref] [PubMed]
- Daouda D, Mbengue M, Bassene ML, et al. Esophageal and Gastric Cancers in Sub-Saharan Africa, Epidemiological and Clinical Review. J Gastroint Dig Syst 2013;S6:007.
- Shin A, Kim J, Park S. Gastric Cancer Epidemiology in Korea. J Gastric Cancer 2011;11:135-40. [Crossref] [PubMed]