Age and factors associated with access and time to post-operative adjuvant chemotherapy in colon cancer: a French epidemiological study
Introduction
Colon cancer (CC) is a major public health problem with 1.4 million cases and more than 600,000 deaths per year worldwide (1). It is the 2nd leading cause of cancer mortality in France (2) and Europe (3), accounting for more than 17 000 deaths each year in France (2). Colectomy remains the cornerstone of treatment and post-operative adjuvant chemotherapy (AC) significantly decreases recurrence and improves survival among patients with high risk stage II (4,5) or stage III colon cancer (5-8). In clinical trials, AC is generally initiated within six to eight weeks after surgery. However, in daily practice, AC is often delayed to more than eight weeks after surgery (9-11) with a negative prognostic impact (9,10,12-16). In addition, older age is associated with fewer AC prescriptions (17,18), despite the fact that chemotherapy is effective in older patients (19-21). Programs in several countries aim to reduce delays and improve access to AC (22,23), but little is known about the associated factors. The objective of our study was to investigate the role of age and other non-organizational factors on access or time to AC in CC patients.
Methods
Study population
We selected cases from our hospital discharge regional database for this multicenter, retrospective, observational, epidemiological study using an algorithm. All adult patients who underwent surgery for stage II or III CC in an authorized health facility for digestive cancer surgery in the “Région Centre-Val de Loire” between January 1 and December 31, 2013, were included. Patients with rectal cancer, a past history of CC, or who received pre-operative chemotherapy were excluded. All data from the medical records were exhaustively included for each health facility.
Data collection
We collected the following data from the patient medical record for each case included in the study: time to AC and likely non-organizational associated factors including age, employment situation, marital status, and medical factors such as the circumstances of diagnosis, emergency or elective surgery, and postoperative morbidities. The study was approved by the CNIL (the French data protection authorities) and CCTIRS (French Advisory Committee on Information Processing in Material Research in the Field of Health), decision DR-2014-132.
Statistical analyses
The populations with and without AC were compared using the Chi square or Student’s test depending on the type of variables. After selection of the relevant variables (P<0.1 by univariate analysis), a multivariate logistic regression model was used to identify factors associated with access to AC.
We studied the time between colectomy and AC for the group that received AC. We excluded the extreme values to calculate the mean time between colectomy and AC, but not for calculating the median. Probable associated non-organizational factors were tested in a univariate linear regression model to select the variables (P<0.1). We then performed a multivariate analysis using a linear regression generalized model (LGM) to identify factors associated with increased time between colectomy and AC. Sex, regional department, and type of health facility (public or private) were included as adjustment variables. Regression coefficients were calculated using SAS 9.3 (SAS Institute).
Results
Population characteristics
Twenty-three of the 24 health facilities of the “Région Centre-Val de Loire” participated in this study from which we identified 914 CC cases from our regional hospital discharge database. Among these, 167 (18%) cases were excluded, mostly due to non-colonic cancer localization (n=104) or algorithm selection errors. Among 747 remaining patients, 404 colectomy cases for stage II (n=240, 59%) or III (n=164, 41%) CC were identified and included in the study (Figure 1).
Characteristics of the population are shown in Table 1. Stage II CC patients received AC significantly less often than stage III CC patients (P<0.0001). Patients receiving post-op AC were significantly younger than those not receiving AC (mean age of 67.6 vs. 77.9 years, P<0.0001). This difference was greater in the stage III only population (mean age of 69 vs. 82.4 years, P<0.0001). Patients who did not receive AC were more often not living as a couple and were retired.
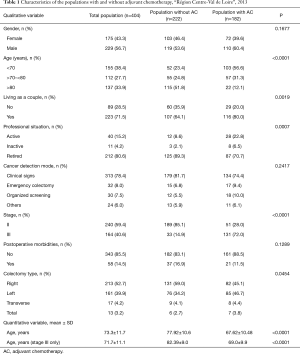
Full table
Factors associated with access to post-operative chemotherapy are shown in Table 2. In multivariate analysis, adjusted for the regional departments and type of health facility (public or private), only two factors were associated with not receiving AC: older age (P<0.0001) and cancer stage (P<0.0001).
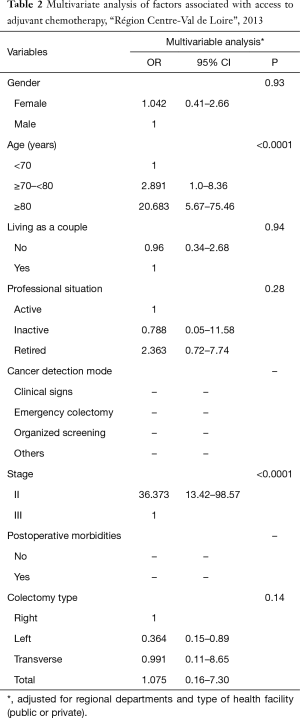
Full table
Time to adjuvant chemotherapy
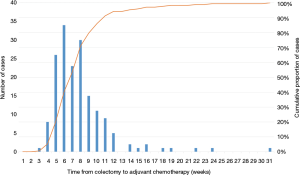
Among the patients receiving AC, the mean time between colectomy and AC was 48 days (SD =14.2). The time to AC exceeded 42 days in 60% of the cases (Figure 2), 56 days in 29%, and 84 days in only 6% (Table 3).
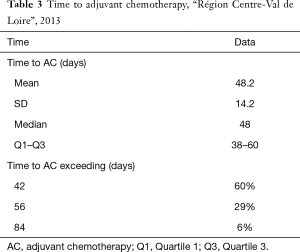
Full table
There was an association by univariate analysis between time to AC and living as a couple, mode of cancer detection, postoperative morbidities and type of colectomy. Age and colon cancer stage were not associated with increased time to receiving AC (Table 4).
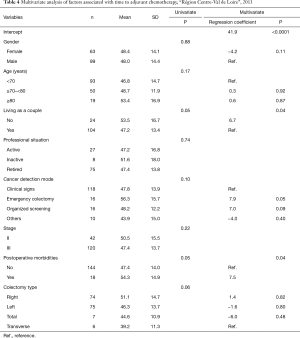
Full table
Three factors were associated with increased time to AC by multivariate analysis: not living as a couple (+6.7 days, P=0.04), cancer detected during an emergency colectomy compared to clinical signs (+7.9 days, P=0.05), and the presence of postoperative morbidities (+7.5 days, P=0.04) (Table 4).
Discussion
Our study addressed colon cancers eligible for colectomy and focused on patient-dependent factors associated with access and time to AC, two factors that have a prognostic impact. Few studies have focused on these two aspects in the same population.
We included 404 patients who underwent surgery for stage II or III colon cancer, in the “Région Centre-Val de Loire” of France. We selected patients based on a hospital discharge database which covered all colectomies for colon cancer in 23/24 authorized health facilities participating in the study. For each case, access to the medical record made it possible to collect all available data.
The presence of missing values for socio demographic variables (i.e., living as a couple or not, professional situation, occupational status), due to the retrospective analysis, led to a statistical loss of power but underlines the strength of our significant findings.
Age was significantly associated with less access to AC (P<0.0001), even for stage III colon cancer where AC is strongly recommended in guidelines (24,25). This finding is in accordance with other studies (17,18,26,27). Similarly, the times to AC are similar to those found in the French national study (by the National Cancer Institute), indicating that our regional sample is representative of the CC population.
We found that some patient characteristics are associated with access (age) and time (not living in couple, emergency colectomy and postoperative morbidities) to AC. Measures implemented in several countries to increase access or shorten the time to AC mostly focus on organizational factors which are identified by studying the functioning of each health facility. These measures are unlikely to modify the factors identified in our study, which should be taken into account when evaluating the impact of organizational measures. Finally, age was a factor of that affected access to AC rather than the time to AC. This aspect requires further study and an appropriate oncogeriatric approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the CNIL (the French data protection authorities) and CCTIRS (French Advisory Committee on Information Processing in Material Research in the Field of Health), decision DR-2014-132.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Institut National du Cancer. Les cancers en France - édition 2015 [Internet]. Available online: http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Les-cancers-en-France-Edition-2015
- Ait Ouakrim D, Pizot C, Boniol M, et al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ 2015;351:h4970. [Crossref] [PubMed]
- Benson AB 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408-19. [Crossref] [PubMed]
- Marshall JL, Haller DG, de Gramont A, et al. Adjuvant Therapy for Stage II and III Colon Cancer: Consensus Report of the International Society of Gastrointestinal Oncology. Gastrointest Cancer Res 2007;1:146-54. [PubMed]
- Benson AB. Adjuvant chemotherapy of stage III colon cancer. Semin Oncol 2005;32:S74-77. [Crossref] [PubMed]
- Macdonald JS. Adjuvant therapy of colon cancer. CA Cancer J Clin 1999;49:202-19. [Crossref] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352-8. [Crossref] [PubMed]
- Bayraktar UD, Chen E, Bayraktar S, et al. Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma? Cancer 2011;117:2364-70. [Crossref] [PubMed]
- Czaykowski PM, Gill S, Kennecke HF, et al. Adjuvant chemotherapy for stage III colon cancer: does timing matter? Dis Colon Rectum 2011;54:1082-9. [Crossref] [PubMed]
- Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer 2006;107:2581-8. [Crossref] [PubMed]
- Bayraktar S, Bayraktar UD, Rocha-Lima CM. Timing of adjuvant and neoadjuvant therapy in colorectal cancers. Clin Colorectal Cancer 2010;9:144-9. [Crossref] [PubMed]
- Berglund A, Cedermark B, Glimelius B. Is it deleterious to delay the start of adjuvant chemotherapy in colon cancer stage III? Ann Oncol 2008;19:400-2. [Crossref] [PubMed]
- Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335-42. [Crossref] [PubMed]
- Chau I, Cunningham D. Adjuvant therapy in colon cancer—what, when and how? Ann Oncol 2006;17:1347-59. [Crossref] [PubMed]
- Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010;46:1049-55. [Crossref] [PubMed]
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091-7. [Crossref] [PubMed]
- Sanoff HK, Carpenter WR, Stürmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624-34. [Crossref] [PubMed]
- Muss HB, Bynum DL. Adjuvant Chemotherapy in Older Patients With Stage III Colon Cancer: An Underused Lifesaving Treatment. J Clin Oncol 2012;30:2576-8. [Crossref] [PubMed]
- Sanoff HK, Goldberg RM. Colorectal Cancer Treatment in Older Patients. Gastrointest Cancer Res 2007;1:248-53. [PubMed]
- Schrag D, Cramer LD, Bach PB, et al. Age and Adjuvant Chemotherapy Use After Surgery for Stage III Colon Cancer. J Natl Cancer Inst 2001;93:850-7. [Crossref] [PubMed]
- Action Cancer Ontario. Ontario Cancer Plan 2008-2011 [Internet]. Available online: https://www.cancercare.on.ca/
- Department of Health. The national cancer strategy [Internet]. Available online: https://www.gov.uk/government/publications/the-national-cancer-strategy
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264:1444-50. [Crossref] [PubMed]
- Adjuvant therapy for patients with colon and rectum cancer. Consens Statement 1990;8:1-25. [PubMed]
- Gilbar P, Lee A, Pokharel K. Why adjuvant chemotherapy for stage III colon cancer was not given: Reasons for non-recommendation by clinicians or patient refusal. J Oncol Pharm Pract 2017;23:128-34. [Crossref] [PubMed]
- El Shayeb M, Scarfe A, Yasui Y, et al. Reasons physicians do not recommend and patients refuse adjuvant chemotherapy for stage III colon cancer: a population based chart review. BMC Res Notes 2012;5:269. [Crossref] [PubMed]



