Impact of sarcopenia on outcomes of locally advanced esophageal cancer patients treated with neoadjuvant chemoradiation followed by surgery
Introduction
Sarcopenia, the progressive and generalized loss of skeletal muscle mass and strength, is an independent predictor of clinical outcomes in multiple gastrointestinal cancers (1,2). It differs from cachexia and weight loss, two known predictors of poor outcomes, in that it is independent of changes in weight or loss of adipose tissue (2). Several studies have looked at the association between sarcopenia and outcomes in patients with esophageal cancer; however, despite its reported relevance in medical and surgical oncology, no such data is yet available for its role in radiation therapy.
Determined using readily available CT scans, sarcopenia could be an objective prognostic marker for patient functional status and future outcomes. Radiologically assessed muscle mass has been proposed as an alternative marker for functional status (3). Considering the central role of CT imaging in treatment planning for radiation therapy, extracting prognostic data from already available imaging studies could help inform clinical decision making. Total psoas area (TPA), as measured on a single cross-sectional CT image at the top slice of the L4 vertebral body level, has been used in a number of studies to predict lean muscle mass and has been correlated with sarcopenia (3).
The purpose of our study was to determine whether sarcopenia defined utilizing TPA could be used to predict outcomes in patients with esophageal cancer treated with neoadjuvant chemoradiation.
Methods
Patients and treatment
After IRB approval, an institutional database consisting of esophageal cancer patients treated with neoadjuvant chemoradiation followed by surgery was queried. Of those 77 patients treated at our institution with intensity modulated radiation therapy (IMRT) and image guided radiation therapy (IGRT) from 2008–2012 that had CT imaging including the L4 vertebral body, 56 met inclusion criteria for this study. All patients received IMRT/IGRT utilizing dose painting to a total dose of 56 Gy to gross disease and 50.4 Gy to high risk nodal regions in 28 fractions along with concurrent cisplatin and continuous infusion 5 fluorouracil chemotherapy as per our institutional pathway (4). Acute toxicity was defined as within 3 months of radiation therapy based on CTCAE version 4.
Assessment of skeletal muscle mass
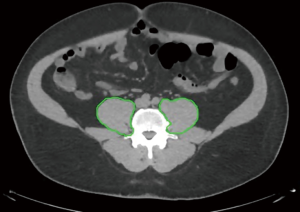
The first full slice of the L4 vertebra alone was identified on axial CT imaging immediately after the L3–L4 interface and the psoas muscle was manually contoured bilaterally as shown in Figure 1 (5). Sarcopenia was defined by the presence of the psoas area less than the median of the cohort. To eliminate measurement bias, all the measurements and calculations were performed by the same investigator (GM). The investigator was trained by a radiation oncologist to identify and measure the psoas muscle area on the first slice of the L4 vertebra immediately after the L3–L4 interface using the treatment planning software (Pinnacle3 TPS version 9.8m, Philips Healthcare, Andover, MA). The volume obtained was then divided by the CT slice thickness for an estimated cross-sectional area and normalized for height (m2) to obtain the L4 skeletal muscle index (mm2/m2) (6).
Statistical analysis
Patient characteristics were evaluated using Pearson Chi-square analysis. Significant dependent variables were then further analyzed using binomial logistic regression. For our specific cohort, receiver operating characteristic (ROC) analysis evaluated TPA with toxicities in order to determine an optimal binomial cutoff. Patient characteristics such as gender, clinical T stage, clinical N stage, and AJCC stage and tumor location were evaluated with univariate analysis, with statistical significance determined at P<0.05. Values significant with univariate analysis were run on multivariate analysis. Kaplan-Meier curves were generated for all survival functions. Cox regression analysis was performed for significant findings. Statistics were run on IBM SPSS 23.0.0.2 64-bit edition (North Castle, NY, USA).
Results
Sarcopenia and clinicopathologic characteristics
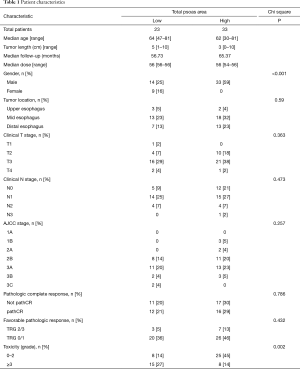
The clinical and pathological characteristics of the 56 patients included in our study are displayed in Table 1. We defined sarcopenia as a TPA less than the median for our cohort, or <841.5 mm2/m2. The mean age of the cohort was 63 (range, 39–81), with a male preponderance (n=47, 84%). Sarcopenia was not significantly associated with tumor location (P=0.59), clinical T stage (P=0.363), clinical N stage (P=0.473), or AJCC staging (P=0.257). Sarcopenia was associated with gender (P<0.001), as shown by all the women in the cohort being sarcopenic.
Full table
Sarcopenia and grade 3 toxicity
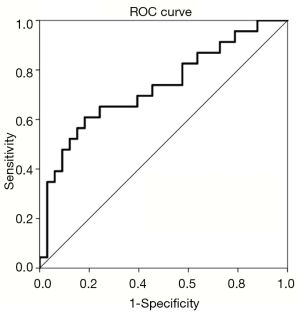
In our cohort of patients, 41% of patients were found to be sarcopenic. We found that the smaller the psoas cross-sectional area, the higher the chance of any grade ≥3 toxicity. In the sarcopenic group, 15 out of 23 patients developed grade 3 or greater toxicities compared to 8 out of 33 patients in the non-sarcopenic group. Among patients with sarcopenia, 10 developed dysphagia that required a feeding tube, 3 developed neutropenia, and 2 were hospitalized during treatment. Among the non-sarcopenic group, 6 patients developed dysphagia requiring a feeding tube, 1 developed radiation pneumonitis, and 1 developed neutropenia. While women in our study were predisposed with a lower TPA, gender stratification with males (P=0.189) and females (P=0.329) did not significantly correlate with toxicities. We saw nearly twice as many grade 3 or greater toxicities in the sarcopenic patient group compared to the non-sarcopenic group. Using the TPA median value of 841.5 (P=0.003, AUC 0.709, sensitivity 60.9%, specificity 78.8%) as the cutoff, sarcopenia was associated with a significant increase in acute grade ≥3 toxicity from chemoradiation on both ROC analysis and logistic regression (P=0.002) as shown in Figure 2. Patients with TPA <841.5 mm2/m2 were 5.78 times more likely to develop a grade 3 or higher toxicity (P=0.004).
Sarcopenia and pathCR and favorable pathologic response
There was no significant association of sarcopenia with pathologic complete response (TRG 0) nor favorable pathologic response (TRG 0/1) based on ROC analysis and logistic regression (P=0.786 and P=0.432 respectively).
Sarcopenia and overall survival
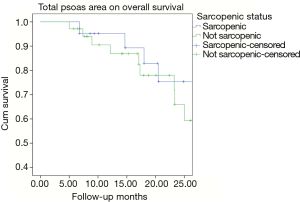
Kaplan-Meier analysis showed no correlation between overall survival (P=0.217) and sarcopenia (TPA) as shown in Figure 3.
Discussion
Progressive and generalized loss of skeletal muscle mass and strength characterizes sarcopenia and has been associated with an increased risk of adverse outcomes (2). In our study we found sarcopenia, as determined by TPA, to be a strong and independent predictor of acute grade ≥3 toxicity (P=0.004, AUC 0.709, OR, 5.78). Grade ≥3 adverse events are of clinical importance and may dramatically affect the patient’s ability to tolerate the completion of tri-modality treatment. In the surgical setting, sarcopenia was an independent predictor of post-operative complications in colorectal, esophageal, pancreatic, and bladder cancers as well as postoperative length of stay and ICU stay in pancreatic cancer (7). Grade ≥3 toxicities experienced were dysphagia requiring feeding tube, any hospitalization, radiation pneumonitis and neutropenia. Patients below our cutoff for sarcopenia were 5.78 times more likely (P=0.004) to develop an acute grade ≥3 toxicity.
The European Working Group on Sarcopenia subdivides sarcopenia by cause into primary and secondary sarcopenia. Primary sarcopenia accounts for the natural process seen as a result of aging in the absence of any other causes of sarcopenia while secondary sarcopenia arises as a result of inactivity, disease, or nutrition (2). Esophageal cancer patients present an overlapping range of phenotypes due to their combination of age, malignancy, and frequent inability to maintain nutritional status as a result of dysphagia caused by their endoluminal tumor burden.
As a result, multiple mechanisms may underlie the development of sarcopenia in these patients. Sarcopenia may reflect the increased metabolic activity of a biologically more aggressive tumor (8). Inflammation, cytokines, myokines, and other processes have all been found to play a role in the development and progression of sarcopenia. The specific influence of each in patients with esophageal cancer is still unknown (2). As skeletal muscle mass decreases and adipose tissue increases, a shift from anti-inflammatory cytokine production towards pro-inflammatory cytokine production occurs (8). This pro-inflammatory state is thought to contribute to the potential for increased postoperative complications and we propose, based on our findings, may play a role in radiation associated toxicities as well (9,10).
In the surgical management of esophageal cancer, the goal of assessing sarcopenia is first to measure the patient’s functional status prior to surgery, but also to determine which patients may benefit from a preoperative intervention while receiving neoadjuvant chemoradiation with reassessment prior to surgery (7). Sheetz et al. confirmed a strong association between frailty, using sarcopenia as a surrogate, and perioperative risk of morbidity among patients who underwent esophagectomy (11). While no consensus has been reached on the optimal intervention for these patients, studies have suggested there are improved surgical outcomes when aggressive nutritional management and implementation of physical therapy for patients with sarcopenia is done preoperatively (8,12). Joglekar et al. in a review of the literature on sarcopenia and its impact within surgical oncology concluded that the prognostic value of sarcopenia on postoperative complications and survival is clinically relevant as it can be objectively and reliably measured (7). Resistance training has been found to be especially beneficial in reversing age-related sarcopenia, however, the practicality of such regimens for cancer patients is not yet established.
In the definitive setting, chemoradiation has been established as superior in outcome to radiation alone for patients with locally advanced esophageal carcinoma, based on doses of 50.4 Gy along with concurrent 5-FU and cisplatin (13). The Intergroup 0123 trial explored dose escalation (64.8 vs. 50.4 Gy with chemotherapy) for improving local control and survival but no difference in 2-year survival, median survival, or 2-year local failure between the high-dose and standard-dose arm was observed (13). This trial has been criticized due to the early deaths in the dose escalation arm before 50.4 Gy, large treatment volumes, and old radiation techniques. Our institutional experience with dose painting to the GTV of 56 Gy while maintaining the 50.4 Gy in 28 fractions to the CTV with IMRT using 4D CT planning and motion management has been shown to be feasible and may be associated with improved rates of pathologic complete or near complete response (4). The data we present here suggests a potential stratification for future trials prospectively exploring dose escalation strategies. Indeed, the ability to modify a patient’s pre-treatment risk factors with improved nutritional and physical conditioning regimens may yield improved patient care and treatment outcomes (7).
Outside of esophageal cancer, sarcopenia has a reported prognostic role in multiple gastrointestinal cancers including gastric, colorectal, hepatic, and pancreatic malignancies (10,14-16) Sarcopenia has also been found to be an independent prognostic factor of outcomes after surgery for cancer in multiple settings (7). For patients receiving radical gastrectomy, sarcopenia was an independent prognosticator for severe postoperative complications, as well as, overall and disease free survival in stage II and III gastric cancer (17). Sarcopenia has been associated with higher morbidity in patients undergoing curative resection for colorectal cancer and shorter disease free and overall survival in patients going to surgery for colorectal liver metastases (10,16). Dodson et al. found that for patients with hepatic malignancies, sarcopenia was associated with an increased risk of overall mortality and worse long term outcomes for patients receiving intra-arterial therapies (15). In pancreatic malignancies, preoperative sarcopenia was found to be an independent risk factor for developing postoperative pancreatic fistulas after pancreaticoduodenectomy (9). In medical oncology, it has also been found to predict worse overall survival and outcomes for patients undergoing chemotherapy for breast, prostate, pancreatic, and renal cancer (14,18-20).
Despite the growing literature in surgical and medical oncology, there has been a paucity of literature exploring the role of sarcopenia in radiation oncology. The current study is hypothesis generating, suggesting not only that an imaging correlate easily obtained at initial staging is associated with toxicity but also that there may be potential for modification with improved nutritional and physical conditioning. CT images are routinely obtained for the diagnosis, staging, and treatment planning of patients diagnosed with esophageal cancer. Using these readily available images to assess sarcopenia provides a valuable, objective prognostic marker for several cancers, specifically esophageal carcinoma in the case of our study (10,21). Available software such as Eclipse (Varian, Palo Alto, CA, USA), has been used to automate the calculation of sarcopenia based on available cutoffs (22). As consensus evolves on the definition of sarcopenia, integration of its calculation into treatment planning systems for radiation therapy would provide prognostic information in real time as a clinician plans patients’ treatments. This could objectively inform a patient centric, individualized approach to radiation dose selection. This work further suggests that sarcopenia may be a stratification tool for selecting those patients potentially most likely to benefit from a SIB approach.
Several limitations of this study should be acknowledged. In their review of sarcopenia’ s impact in surgical oncology, Joglekar et al. found that while sarcopenia’s broader definition has been widely accepted, there remains no standardized methodology for classifying and assessing sarcopenia within the clinic (7). One commonly used definition sets sex specific cutoffs for men and women based on a Canadian cohort of 250 patients with thoracic and GI cancers (5). Another definition sets cutoffs based on a cohort of 1,000 cancer patients with lower BMIs and is commonly used for studies in Asian populations due to the differences in body composition between eastern and western populations (23). Even still, other studies have used tertiles and quartiles to determine cutoffs for sarcopenia (7).
The European Working Group on Sarcopenia in Older Populations recommends using the presence of both low muscle mass and low muscle function (strength or performance) for the diagnosis of sarcopenia (2). Due to the retrospective nature of our study, only muscle mass was able to be assessed in this study. We acknowledge that prospective trials assessing function through handgrip strength and the timed get up and go test would provide added validity to the assessment of sarcopenia in future studies (2). In patients with esophageal cancer, weight loss due to dysphagia may lead to a decrease in muscle mass and, consequently, to an overestimation of sarcopenia, potentially decreasing its sensitivity in this disease site (24).
Future studies should evaluate assessment of sarcopenia guidelines to provide cut-offs for reliable and reproducible prognostic value across institutions and potentially disease sites. We contend that cutoffs for sarcopenia may even vary in their prognostic value across disease sites and the cutoffs conferring prognostic value for one malignancy may differ from that of another. As our project was a retrospective cohort study, we recognize the need for consensus guidelines to inform future large scale, prospective, or multi-institutional trials to validate our findings due to the small size of our cohort and single institutional sampling. Further prospective validation of our data could improve patient selection and risk stratification for aggressive preoperative nutritional management and implementation of physical therapy. These interventions may lead to improved outcomes and reduced morbidity.
In conclusion, sarcopenia is an integrated and quantitative marker of frailty (9). Although we found no difference in pathologic response or overall survival in patients with sarcopenia receiving neoadjuvant chemoradiation, sarcopenia was associated in this study with a significant increase in acute grade ≥3 toxicity. This suggests a potential role for its use in guiding neoadjuvant patient selection strategies. Pre-therapy assessment of sarcopenia could provide objective guidance as to which patients are candidates for dose escalation, which patients may need additional supportive and nutritional management, and which patients are less likely to tolerate the full course of tri-modality treatment. Improved nutrition and strength training leading to improved muscle mass in the three-month period leading up to esophageal resection could improve not only toleration of neoadjuvant treatment but also surgical outcomes as well. The potential to integrate sarcopenia measurement from the initial CT at diagnosis into the clinical algorithm and treatment planning software for personalized radiation dose delivery may lead to improved outcomes and requires further prospective clinical evaluation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This study was approved by Institutional Review Board of Moffitt Cancer Center (No. MCC 16567) and informed consent was taken from all the patients.
References
- Wagner D, DeMarco MM, Amini N, et al. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg 2016;8:27-40. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Jones KI, Doleman B, Scott S, et al. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 2015;17:O20-6. [Crossref] [PubMed]
- Shridhar R, Choung MD, Hoffe SE, et al. Effect of neoadjuvant dose-painted IMRT to 56 Gy for locally advanced esophageal cancer on outcomes. J Clin Oncol 2011.29. abstract 144.
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629-35. [Crossref] [PubMed]
- Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997-1006. [Crossref] [PubMed]
- Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J Surg Oncol 2015;112:503-9. [Crossref] [PubMed]
- Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012;4:535-46. [Crossref] [PubMed]
- Nishida Y, Kato Y, Kudo M, et al. Preoperative Sarcopenia Strongly Influences the Risk of Postoperative Pancreatic Fistula Formation After Pancreaticoduodenectomy. J Gastrointest Surg 2016;20:1586-94. [Crossref] [PubMed]
- van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550-7. [Crossref] [PubMed]
- Sheetz KH, Zhao L, Holcombe SA, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 2013;26:716-22. [PubMed]
- Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr 2015;6:452-60. [Crossref] [PubMed]
- Neuner G, Patel A, Suntharalingam M. Chemoradiotherapy for esophageal cancer. Gastrointest Cancer Res 2009;3:57-65. [PubMed]
- Choi Y, Oh DY, Kim TY, et al. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLoS One 2015;10:e0139749. [Crossref] [PubMed]
- Dodson RM, Firoozmand A, Hyder O, et al. Impact of sarcopenia on outcomes following intra-arterial therapy of hepatic malignancies. J Gastrointest Surg 2013;17:2123-32. [Crossref] [PubMed]
- Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol 2015;22:2663-8. [Crossref] [PubMed]
- Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore) 2016;95:e3164. [Crossref] [PubMed]
- Antoun S, Birdsell L, Sawyer MB, et al. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol 2010;28:1054-60. [Crossref] [PubMed]
- Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920-6. [Crossref] [PubMed]
- Galvão DA, Taaffe DR, Spry N, et al. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010;28:340-7. [Crossref] [PubMed]
- Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115-22. [PubMed]
- Grendarova P, Arora R, Bebb GD, et al. Sarcopenia Is Associated With Worse Overall Survival in Patients With Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;96:S201-S202. [Crossref]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Makiura D, Ono R, Inoue J, et al. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: A retrospective cohort study. J Geriatr Oncol 2016;7:430-6. [Crossref] [PubMed]