The International Duration Evaluation of Adjuvant Chemotherapy study: implications for clinical practice
After long anticipation, the preliminary results of the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) study were reported at the American Society of Clinical Oncology on June 4, 2017. These results offer some guidance to the practicing medical oncologist on the duration of adjuvant therapy for stage III colon cancer. Yet, the results create more questions than answers and provide significant challenges when facing treatment decision for adjuvant therapy based on stage, age, performance status, and primary site. In this perspective, we reflect on the recent reported data and its potential impact on day-to-day practice.
It has been recognized that 9 or 12 months of adjuvant fluoropyrimidine therapy are no better than 6 months in stage III colon cancer; however, little guidance existed on more abbreviated courses of adjuvant therapy (1,2). Chau et al. conducted and reported on a randomized clinical trial of 12 weeks of continuous infusion 5-FU in comparison to a control arm of bolus 5-FU/LV as per Mayo Clinic regimen for 6 months (3). Surprisingly, 12 weeks of adjuvant protracted 5-FU infusion therapy resulted in a numerically superior (statistically insignificant) disease free survival (DFS) and overall survival (OS) in comparison to 6 months of 5-FU/LV. This infusional regimen was not adopted into clinical practice for several reasons: (I) the study was a superiority study that did not reach its primary endpoint; (II) the study suffered from significant patient heterogeneity and included patients with stage II and III colon and rectal cancers; (III) no other supporting studies confirmed similar findings. In addition, retrospective analyses of Medicare data base had shown an increase in colon cancer-specific death rate in patients receiving 4 months or less of adjuvant fluoropyrimidine therapy in comparison to 5–7 months, therefore suggesting the need to abide with a 6-month regimen (4). However, the question of shorter adjuvant therapy took central stage again as oxaliplatin was incorporated in the routine adjuvant treatment of stage III colorectal cancer. Three phase III clinical trials had shown that 6 months of adjuvant oxaliplatin plus a fluoropyrimidine is superior to 6 months of adjuvant fluoropyrimidine alone in stage III colon cancer (5-7). These DFS improvements translated in clinically modest but statistically significant improvements in OS on the oxaliplatin arms on the MOSAIC and AVANT clinical trials, while a trend towards a statistical significance was noted on the NSABP trial. The NSABP FLOX (bolus 5-FU/LV plus oxaliplatin) regimen incorporated only 75% of the total dose of oxaliplatin on MOSAIC or AVANT trials. In addition, all three trials reported a compromised dose intensity in oxaliplatin secondary to bone marrow and neurological toxicities (Oxaliplatin dose intensity: MOSAIC =80%; NSABP =88%; AVANT =84%). All three studies reported an increased incidence of grade 3 neuropathy (MOSAIC =12%; NSABP =8.2%; AVANT =11%) which remained clinically significant in more than 10% of patients at 2 years (5-8). Given the clinical relevance of oxaliplatin-induced neuropathy, the uncertainty regarding the optimal duration of oxaliplatin treatment, and the low rate of oxaliplatin-associated neuropathy when treatment is limited to 12 weeks, several cooperative groups embarked on conducting randomized phase III clinical trials to test 3 months of oxaliplatin plus fluoropyrimidine vs. 6 months of oxaliplatin plus fluoropyrimidine in the adjuvant setting of stage III colon cancer.
The IDEA study in a plenary session at ASCO 2017
The IDEA collaboration prospectively collected and pooled results from six randomized clinical trials [SCOT, TOSCA, Alliance/SWOG 80702, IDEA France (GERCOR/PRODIGE), ACHIEVE, HORG] from 12 countries to assess if the DFS with 3 months of adjuvant oxaliplatin and fluoropyrimidine is non-inferior to 6 months in patients with stage III colon cancer (9). The studies allowed the use of either FOFLOX or capecitabine plus oxaliplatin (CAPOX) as adjuvant therapy, except CALGB/SWOG trial which mandated the use FOLFOX in all patients. CALGB/SWOG, IDEA France, and ACHIEVE studies were limited to stage III colon cancer, while the other studies included stage II patients. Only the SCOT trial allowed rectal cancer patients to participate (Table 1). The IDEA combined analysis was limited to stage III colon cancer patients. Non-inferiority was to be declared if the upper limit of the 95% confidence interval (CI) of the hazard ratio (HR) for DFS was less than 1.12. If satisfied, this would have indicated that there is less than 5% chance that 3 months of adjuvant therapy results in a relative decrease in DFS that exceeds 12% when compared to 6 months of adjuvant therapy (9). A planned accrual of 10,500 was needed to allow adequate power to answer the non-inferiority question. A total of 12,834 patients with stage III colon cancer enrolled between June, 2007 and December, 2015 were the subject of analysis, with results reported as a Late Breaking Abstract at the plenary session of ASCO 2017 (9). Approximately 40% of patients received adjuvant CAPOX and the rest received FOLFOX (FOLFOX4 or mFOLFOX6). High risk pathology including T4 and N2 disease occurred in 21% and 28%, respectively. At a median follow-up of 39 months, 3,263 (25.4%) DFS events were reported. The 3-year DFS for the 3 months adjuvant chemotherapy group was 74.6% vs. 75.5% for the 6 months adjuvant chemotherapy group. The HR for DFS for 3 months of chemotherapy was 1.07 (95% CI: 1.00–1.15). The upper boundary of the CI was 1.15, exceeding the set point of 1.12 and therefore failing to confirm non-inferiority. However, a pre-planned analysis of DFS by treatment arm revealed significant differences between the FOLFOX and CAPOX arms. The HR for DFS for 3 vs. 6 months was 1.16 (95% CI: 1.06–1.26) and 0.95 (95% CI: 0.85–1.06) for FOLFOX and CAPOX, respectively. A statistically significant interaction between DFS and regimen was noted, confirming a difference in outcome by treatment regimen (FOLFOX vs. CAPOX). This discordance was consistent across all clinical trials that contributed to the IDEA project, except the Greek HORG trial—which contributed the lowest number of patients to this analysis.

Full table
The T4 or N2 (high risk group) represented 41% of the population and had a remarkably worse DFS outcome than the lower risk T1-3N1 group (3-year DFS: 63% vs. 83%). A pre-planned analysis of outcome by T and N stage failed to show a statistically significant interaction P value, although the T4 or N2 stage disease showed a numerically higher HR of 1.15 (95% CI: 1.03–1.23) for 3 vs. 6 months therapy in comparison to the HR of 1.01 for the T1-3 N1 group (95% CI: 0.9–1.12). While both HR overlap with the pre-set 1.12 limit, these data suggest that the clinical benefit from extending oxaliplatin-based therapy from 3 to 6 months of chemotherapy are minimal, if existing, for the T3N1 patients. Although not a pre-planned analysis, the investigators evaluated the outcome of the low-risk T3N1 and high-risk T4 or N2 groups by FOLFOX and CAPOX regimens, separately (Table 2). The HR for CAPOX 3 vs. 6 months were 0.85 and 1.02 for the low and high-risk groups, respectively. The HR for FOLFOX 3 vs. 6 months were 1.1 and 1.2 for the low and high-risk groups, respectively.
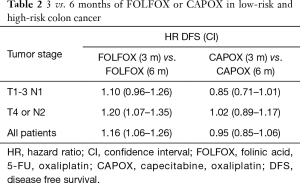
Full table
What is the take home message from the IDEA study?
While the study failed its primary non-inferiority endpoint, there are important takeaways from this analysis. There is a difference in outcome between 3 vs. 6 months of adjuvant therapy by treatment arm. This is seen on the IDEA combined analysis and demonstrated in 5 of the 6 clinical trials that constituted the IDEA data set. The interaction between treatment and DFS is statistically significant.
Three months of CAPOX was non-inferior to 6 months of CAPOX in the overall IDEA stage III colon cancer patient population (HR =0.95). When stratifying by risk groups, the HR for 3 vs. 6 m for CAPOX were 0.85 and 1.02, respectively. While this analysis was not pre-planned, the large data set analyzed gives me a comfort level to limit CAPOX to 3 months for lower risk stage III colon cancer patients. There are no perceived advantages in this subgroup from extending oxaliplatin therapy, especially given the differential in neuropathy that is noted between 3 vs. 6 months of treatment (Grade 2–4: 17% vs. 48%). What about CAPOX in higher-risk patients? Should CAPOX be continued for 6 months in this subgroup of patients? Could the benefit from CAPOX trump the associated toxicity? The IDEA study noted a HR of 1.02 (95% CI: 0.89–1.17) for T4 or N2 high-risk patients, crossing the pre-specified 1.12 mark. Therefore, one should not conclude that 3 months of CAPOX is equivalent to 6 months in the high-risk population. However, the degree of benefit from extending adjuvant CAPOX by 3 months in this high-risk group appears to be limited based on the reported 1.02 reported HR and associated CIs. Patients and physicians should balance the limited potential DFS benefit and the increased toxicities when opting for 6 months adjuvant CAPOX therapy in the high-risk group.
Three months of FOLFOX was not non-inferior to 6 months of FOLFOX in the overall IDEA stage III colon cancer patient population (HR =1.16). Furthermore, non-inferiority of 3 vs. 6 months could not be established for the low risk stage III population (HR =1.10; 95% CI 0.96–1.26) while evidence of harm was noted in the high-risk population (HR =1.20; 95% CI 1.07–1.35). This means that the incremental benefit for low-risk stage III disease at 3 years is less 2% while the incremental benefit for high risk disease neighbors 8%. Six months of FOLFOX remains the recommended duration of adjuvant therapy in high risk stage III disease. While 3 months of FOLFOX was not proven to be non-inferior to 6 months in the low-risk stage III population, the benefits of extending FOLFOX to 6 months are clinically limited and this should be weighed against the increased risk of neuropathy.
Are FOLFOX and CAPOX different when it comes to adjuvant therapy?
The discordance in outcome with CAPOX and FOLFOX on the IDEA trial was not anticipated. Since treatment choice was non-randomized and was based on the investigator’s choice, one cannot directly compare outcomes between CAPOX and FOLFOX. Yet, one can speculate on the potential reasons for the discrepancy of the 3 and 6 months outcomes between these two backbones. One potential explanation may be related to the potential superiority of capecitabine over fluorouracil in the adjuvant setting. It is possible that capecitabine’s optimal benefits require 3 months of adjuvant treatment while 5-FU requires 6 months. Indeed, Chau et al. reported the equivalence of 12 weeks protracted 5-FU, a regimen that mechanistically mimics capecitabine therapy, to 6 months of bolus 5-FU/LV (3). In addition, the XACT clinical trial had previously reported a superior relapse free survival and a strong trend towards superiority of DFS with capecitabine vs. fluorouracil in stage III colon cancer (including N1 and N2 disease) (10). Therefore, one may hypothesize that 3 months of CAPOX is sufficient for the adjuvant treatment of stage III colon cancer and that 6 months of FOLFOX are needed to result in the same benefits. This hypothesis is contradicted by lack of differences in the activity of CAPOX vs. FOLFOX in the metastatic disease setting, a small negative randomized phase III clinical trial of CAPOX vs. FOLFOX in the adjuvant treatment of stage II and III colon cancer, and similar advantages in terms of DFS HR across three randomized trials comparing oxaliplatin plus capecitabine or oxaliplatin plus 5-FU/LV to 5-FU/LV control backbones (5-7,11,12).
Alternatively, it is possible that differences in dose modifications in the setting of FOLFOX and CAPOX result in different treatment effects, leading to lesser efficacy for CAPOX than FOLFOX in the 6 months regimens. This may have resulted from an incremental benefit from continuing FOLFOX but not CAPOX beyond the 3 months interval. Indeed 71% of patients receiving FOLFOX reached the last cycle of treatment while 65% of patients receiving CAPOX did. In addition, the IDEA investigators reported at ASCO a numerically higher dose intensity for 5-FU (81.6%) than capecitabine (78%) on the FOLFOX and CAPOX arms, respectively. Similarly, a slightly higher dose intensity of oxaliplatin was administered on the FOLFOX arm (72.8%) than the CAPOX arm (69.3%). The differential in dosing in the 6 months arms of FOLFOX and CAPOX may therefore explain the increased reported grade 3–4 neurotoxicity seen with FOLFOX vs. CAPOX (16% vs. 9%). While this hypothesis is intriguing, it is highly unlikely given the relatively minor differences noted in dose intensity. In addition, one would have at least seen a trend towards some benefit with 6 vs. 3 months for CAPOX, an event that was not apparent.
How does this impact my practice?
Given the miniscule benefit in 3-year DFS in T1-3N1 patients in the pooled analysis, one can certainly consider 3 months of FOLFOX or CAPOX as an adjuvant treatment for this group of patients. Such a consideration is warranted considering the differential in neurotoxicity rates between 3 and 6 months, especially that neurotoxicity can be a life-changing long term adverse event in some patients. Given the lack of inferiority with a CAPOX regimen (which was not confirmed for FOLFOX), I may favor this combination in patients without contraindications to capecitabine-based therapy. It is important to note that the dosing of capecitabine on the IDEA trials was 1,000 mg/m2/dose BID ×14 days every 21 days. Given the curative intent of adjuvant therapy, I would not recommend a lower starting dose. US oncologists routinely use an 850 mg/m2/dose BID ×14 days every 21 days in settings of CAPOX therapy based on the TREE clinical trials (13). The efficacy of this ameliorated dosing in adjuvant settings has not been confirmed and should not be recommended at this time. I am also not ready to extrapolate these recommendations to all T1-3N1 patients. Patients with high grade tumors, pericolonic tumor implants, and extranodal disease extension have a higher risk of disease relapse and may not qualify for a “low-risk” group stratification. While we await further subgroup analyses from the IDEA trial, these patients should still be considered for treatment in a similar fashion as high-risk stage III colon cancer.
For patients with high risk (T4 or T1-3N2) stage III colon cancer, one should still recommend 6 months of adjuvant therapy. It is unclear if CAPOX or FOLFOX have different outcomes in such settings and the decision should be individualized. I personally find FOLFOX better tolerated than CAPOX and I will likely find myself continuing to offer this 6 months regimen to many of my high-risk patients.
More questions remain
The IDEA trial renders some support to 3 months oxaliplatin-based therapy for patients with low risk stage III disease. However, it is important to note that this data cannot and should not be extrapolated to adjuvant therapy with fluoropyrimidine monotherapy. Patients considered for capecitabine of 5-FU monotherapy adjuvant treatment should still receive 6 months of chemotherapy, irrespective of their risk status. In addition, it will be difficult to apply this data to adjuvant rectal cancer patients. Most locally advanced rectal cancer patients currently receive neoadjuvant chemoradiation followed by surgery and then 4 months of FOLFOX chemotherapy. Knowing the difficulty of conducting a repeat non-inferiority study in patients with rectal cancer, it may be reasonable to consider 3 months of oxaliplatin based therapy in patient with excellent pathological down-staging and yN0 tumors. Some extrapolation to cases where total neoadjuvant therapy is applied may also be needed. A consideration for a 3-month rather than a 4-month neoadjuvant chemotherapy with oxaliplatin-based therapy prior to chemoradiation and surgery will be reasonable in cases with clinical stage II disease or T1-T3N1 disease. As far as resected metastatic disease, and given the excessive risk of relapse in this patient population, I will not consider any changes in my perioperative or adjuvant chemotherapy practice: 6 months of oxaliplatin-based therapy is still recommended.
The IDEA study is one step in the right direction. Thousands of patients will be spared severe neuropathy with the implementation of short term oxaliplatin-based therapy in the appropriate stage III population. Minimizing unwarranted chemotherapy exposure in other colorectal risk groups should be a subject of active investigation. Further progress can only be achieved through additional large-scale studies that are associated with predictive and prognostic biomarker assays. For the time being, consensus guidelines are urgently needed to address the many questions raised by IDEA project.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005;23:8671-8. [Crossref] [PubMed]
- Andre T, Colin P, Louvet C, et al. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol 2003;21:2896-903. [Crossref] [PubMed]
- Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol 2005;16:549-57. [Crossref] [PubMed]
- Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol 2006;24:2368-75. [Crossref] [PubMed]
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. [Crossref] [PubMed]
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [Crossref] [PubMed]
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. [Crossref] [PubMed]
- Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 2007;25:2205-11. [Crossref] [PubMed]
- Shi Q, Sobrero A, Shields A, et al. Prospective pooled analysis of six phase III trials investigating duration of adjuvant (adjuv) oxaliplatin-based therapy (3 vs 6 months) for patients (pts) with stage III colon cancer (CC): The IDEA (International Duration Evaluation of Adjuvant chemotherapy) collaboration. J Clin Oncol 2017;35:abstr LBA1.
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. [Crossref] [PubMed]
- Pectasides D, Karavasilis V, Papaxoinis G, et al. Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer. BMC Cancer 2015;15:384. [Crossref] [PubMed]
- Arkenau HT, Arnold D, Cassidy J, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. J Clin Oncol 2008;26:5910-7. [Crossref] [PubMed]
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and Efficacy of Oxaliplatin and Fluoropyrimidine Regimens With or Without Bevacizumab As First-Line Treatment of Metastatic Colorectal Cancer: Results of the TREE Study. J Clin Oncol 2008;26:3523-9. [Crossref] [PubMed]


