Survival benefit of neoadjuvant versus adjuvant radiotherapy in lymph node positive esophageal cancer: a population based analysis
Introduction
Esophageal cancer (EC) is an aggressive and challenging disease with very poor prognosis (1). It is the 5th most common gastrointestinal cancer in the US, accounting for about 1% of all cancers diagnosed every year (2). According to American Cancer Society, about 17,000 new cases were reported in 2015, resulting in 15,590 deaths (2).
Histology of EC is dependent on its location, with squamous cell carcinoma (SCC) being more prevalent in the upper and middle thirds of the esophagus, and adenocarcinoma (AC) in the lower third of the esophagus and at the gastroesophageal junction. SCC is the most common EC type worldwide and is predominantly diagnosed in patients from Middle East and central and eastern Asia. However, a noticeable increase in incidence of lower esophageal AC accompanied by a decline in SCC was recently noted in Western countries (3,4). This upward trend is attributed to changes in lifestyle, including increasing incidence of obesity and the associated gastroesophageal reflux disease.
Although surgery is the traditional treatment for EC, it exhibits modest efficacy, with 5-year survival rate that typically does not exceed 30% (5). These dismal results have led to the development of multimodal therapy involving a combination of several treatment types, such as surgery, chemotherapy and radiotherapy (RT). The current National Comprehensive Cancer Network (NCCN) guidelines recommend neoadjuvant or definitive chemoradiation for all patients for whom the disease stage exceeds T1N0 (6). However, EC treatment remains non-uniform, due to the lack of specific recommendations on the timing of radiotherapy in relation to surgery.
Review of pertinent literature reveals reports on several randomized trials as a part of which the survival benefit of preoperative RT and post operative RT was evaluated with highly inconsistent results. Randomized trials by Launois et al., Wang et al., Nygaard et al. failed to establish superiority of preoperative RT versus surgery alone (7-9). Similarly, Fok et al., Ténière et al. reported no difference in survival when patients received postoperative RT in addition to surgery (10,11). However, when interpreting these findings, it is essential to note that the aforementioned results pertain to trials conducted before 1992, when treatment strategies were more rigid (12). Also majority of the patients involved in these trials had SCC. Consequently, the findings yielded by earlier studies are no longer applicable. In addition, as the survival advantage of neoadjuvant vs. adjuvant RT in lymph node (LN) positive patients with EC has not been examined, further research in this field is needed. To fill this gap in the extant knowledge, in this work, we retrospectively analyzed data sourced from Surveillance, Epidemiology, and End Results (SEER) Registry, a US population-based database, to ascertain if there are any differences in survival outcomes among patients with LN positive EC who were treated with neoadjuvant RT vs. adjuvant RT.
Methods
In 1973, National Cancer Institute established the SEER program, thereby creating a comprehensive dataset that holds information on cancer diagnosis, incidence, survival and treatment modalities. This data is collected from 18 population-based registries representing approximately 26% of the US population (13).
For this study, using International Classification of Diseases for Oncology, 3rd Edition (ICD-0-3), topographical codes (C1 5.0–15.9) and morphological codes (histology/behavior: 8050/3, 8070/3, 8140/3, 8260/3, 8030/3, 8261/3, 8480/3), we searched the SEER database aiming to identify patients that were diagnosed with non-metastatic LN positive EC between 2004 and 2007 who underwent resection. The data pertaining to these patients was retrospectively analyzed to obtain information regarding patient demographics (age, sex, race), tumor characteristics (histology, size, extent of disease, nodal involvement) and treatment modalities (surgery, timing of radiation treatment relative to surgery and type of radiation received). We used American Joint Committee on Cancer (AJCC) 6th edition published in 2004 to determine nodal involvement and disease extent. Finally, the patients who met the aforementioned inclusion criteria were divided into two groups according to the timing of RT relative to surgery (neoadjuvant vs. adjuvant).
Since the data from SEER did not include any patient identifying information, Institutional Review Board approval was not required.
Statistical analysis
In the present study, 5-year relative survival rate was the primary outcome. Kaplan-Meier method was employed to calculate survival rates and curves. Log-rank test and Cox proportional regression model were used to conduct univariate and multivariate analysis, respectively. Relative 5-year survival rate was calculated and compared using the SEER*Stat software version 8.4.1. In addition, IBM SPSS version 20 was used to generate Kaplan-Meier curve for each group (P<0.05 was considered as statistically significant).
Results
Patient characteristics
A total of 11,572 patients were diagnosed with EC in 2004–2007 and 22.4% (2,597 patients) had LN involvement without distant metastasis; 933 patients met the previously delineated inclusion criteria (Figure 1). They were separated into two groups, including 636 and 297 patients, based on whether they received RT before or after the surgery. Demographic characteristics of the patients in both groups are outlined in Table 1.
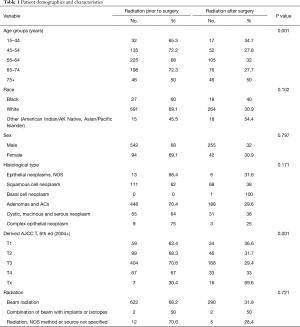
Full table
Majority of the patients were aged 55 to 64 years (60%), white (91.6%) and male (85.4%). At 68.2%, adenomas and AC were the most frequent histological type followed by SCC at 19.1%. T3 was the most common stage (61.3%) in both groups. 97.7% of the patients received beam radiation.
Survival effects of various prognostic factors in both groups are listed in Table 2. Our analyses revealed no significant differences in the overall 5-year survival rate between patients who received RT prior to surgery relative to those that received RT after surgery (32.8% vs. 26.5%, P=0.058). Moreover, statistically significant differences in relative 5-year survival rates of patients with T3 and Tx stage of EC were noted between neoadjuvant and adjuvant RT groups (T3 28.5% vs. 20.2%, P=0.011; Tx 46.3% vs. 8.9%, P=0.021) (Figure 2). Our findings further revealed statistically significant differences in relative 5-year survival rates for males (33.2% vs. 25.9%, P=0.034) and patients with squamous cell EC (43.4% vs. 26.5%, P=0.035) in neoadjuvant RT relative to adjuvant RT group.
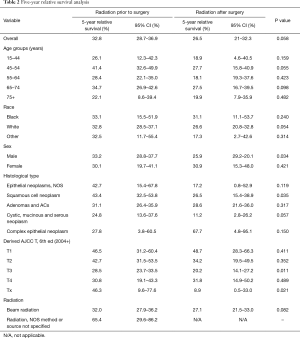
Full table
However, in multivariate analysis, significant increase in the 5-year survival rate was observed only in patients diagnosed with T3 or Tx stage when RT was given in neoadjuvant setting (P<0.05). More specifically, when the survival rates of the two groups were analyzed according to age (P=0.155), race (P=0.06), sex (P=0.054), histological type (P=0.063) and type of radiology (P=0.073), no statistical differences were noted.
Discussion
The role of neoadjuvant and adjuvant RT in esophageal carcinoma outcomes has been controversial for decades, making it difficult for practitioners to recommend the most optimal course of treatment. In most extant studies in this field, effectiveness of preoperative or postoperative RT was assessed using samples comprising solely of patients that underwent surgery (14-17). Moreover, meta-analyses of trials that were conducted more than 2 decades ago revealed no differences in survival rates among patients that were subjected to these two treatment modalities (14,18,19) As survival rates following neoadjuvant vs. adjuvant RT in patients with nodal-positive esophageal carcinoma have not been compared to date, this was the goal of the present investigation.
LN involvement is very common in patients diagnosed with esophageal carcinoma, especially in AC (20). LN involvement is observed in approximately 80% of patients who have T3 tumors, which is an important negative prognostic factor in patients who undergo curative resection (21). However, survival rates in this group of patients have not been assessed in pertinent research.
Our large population-based study focused on this specific group of patients. We revealed greater benefits of neoadjuvant vs. adjuvant RT, especially in male patients with SCC pathology subtype and T3, Tx stage cancer. These findings may assist oncologists and patients in making treatment decisions.
RT when given preoperatively, may downstage tumors, thus making resection easier, with less chance of involving important margins. Consequently, it could increase survival rates relative to surgery alone. Meta-analyses examined as a part of a literature review suggest that this treatment mode may offer a modest benefit to patients, reducing the risk of death by 11%, while improving absolute 2- and 5-year survival from 30% to 34% and 15% to 18%, respectively (9,12,14,21-23).
However, only one meta-analysis involving patients that underwent preoperative radiotherapy alone has been published to date (5). This Cochrane review involved five randomized trials as a part of which outcomes following surgery alone were compared to those when surgery was preceded by preoperative RT. With a median follow-up of nine years, the outcomes reported in these trials revealed an improvement in survival among patients that received combined therapy. The hazard ratio (HR) of 0.89 (95% CI: 0.78–1.01) suggests an overall 11% reduction in the risk of death and an absolute 2- and 5-year survival benefit of 3% and 4%, respectively (12,24). However, in the analyzed samples, SCC was the dominant histology, making these findings less relevant today, given that AC has become more prevalent in the US, with most tumors located in the distal esophagus (23-26). Consequently, the role of radiotherapy in AC, with the potential for more advanced local-regional tumors, is far less clear.
Our study sample primarily comprised of patients with AC, and the results show that, while patients with SCC can benefit more from preoperative radiotherapy, this is not the case for those diagnosed with AC. These findings may indicate that the survival of patients with AC pathology subtype cannot be improved only by local treatment.
Xiao et al. showed that postoperative RT could improve the 5-year survival rate in esophageal carcinoma patients with positive LN metastases, as well as in patients with stage III disease, when compared with similar patients who did not receive radiation therapy (27). According to the reported results, the 5-year survival rates of LN positive patients were 14.7% and 29.2% (P=0.0698), respectively, while 13.1% and 35.1% (P=0.0027) was reported for stage III patients. Although no statistically significant differences were found in the overall 5-year survival rates of these two groups, as 31.7% was reported for surgery group vs. 41.3% for surgery and radiotherapy group (P=0.4474), the role of postoperative radiotherapy in LN positive patients was addressed in this trial.
Although no survival differences between pre- and postoperative RT were reported in several existing studies (5,15,16), when interpreting these findings, it is worth noting that these analyses were typically based on trials conducted before 1992, making them less relevant today, given the advances in treatment options and changes in patient profiles.
Even though our large population-based study revealed that preoperative radiotherapy is more beneficial than postoperative radiotherapy (P=0.024), especially when offered to male patients with SCC pathology subtype and T3 stage, several limitations inherent to the SEER database itself should be noted. Firstly, our results were yielded by a retrospective analysis. The patients were grouped based on treatment mode (neoadjuvant vs. adjuvant radiotherapy) and were thus not randomized, potentially resulting in a selection bias. Secondly, no information about chemotherapy, radiation technologies or specific surgical approach was recorded in the database, making it difficult to ascertain whether the survival benefit should be attributed to chemo-radiotherapy, advanced surgery technology or modern radiotherapy. These aspects require further investigation, given that the radiation therapy technology has rapidly evolved during the past few decades, allowing many tumors located in a wide range of sites to be treated with highly conformal technology, including intensity modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT). The new technologies have enabled doctors to deliver high doses of radiation to the tumor with much greater precision, thus limiting the radiation exposure of the surrounding tissues and organs, thereby dramatically reducing morbidity. As survival rates are dependent on the radiation technologies used, they should also be examined in future studies. Additionally, as an old version of the N stage system was employed in our study, the data was not categorized by the number of LNs, which may limit the potential for generalizing our results beyond the patients included in the sample.
Conclusions
Our findings indicate that, in terms of survival rates, neoadjuvant RT is more beneficial than adjuvant RT to esophageal carcinoma patients who have LN involvement, and especially to male patients with SCC pathology subtype and T3 stage. Clinical trials are needed to further confirm the role of neoadjuvant RT in this group of positive EC patients undergoing curative resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The SEER database does not hold any patient disclosing information. Hence, Patient consent and IRB approval was not required.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Cowie A, Noble F, Underwood T. Strategies to improve outcomes in esophageal adenocarcinoma. Expert Rev Anticancer Ther 2014;14:677-87. [Crossref] [PubMed]
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Available online: https://seer.cancer.gov/archive/csr/1975_2006/
- Esophageal and esophagogastric junction cancers, NCCN guidelines, version 1. 2016. Available online: http://www.nccn.org/
- Launois B, Delarue D, Campion JP, et al. Preoperative radiotherapy for carcinoma of the esophagus. Surg Gynecol Obstet 1981;153:690-2. [PubMed]
- Wang M, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of esophageal carcinoma: report on 206 patients. Int J Radiat Oncol Biol Phys 1989;16:325-7. [Crossref] [PubMed]
- Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg 1992;16:1104-9; discussion 1110. [Crossref] [PubMed]
- Fok M, Sham JS, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138-47. [PubMed]
- Ténière P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123-30. [PubMed]
- Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 2007;8:545-53. [Crossref] [PubMed]
- Wingo PA, Jamison PM, Hiatt RA, et al. Building the infrastructure for nationwide cancer surveillance and control--a comparison between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) Program (United States). Cancer Causes Control 2003;14:175-93. [Crossref] [PubMed]
- Schwer AL, Ballonoff A, McCammon R, et al. Survival effect of neoadjuvant radiotherapy before esophagectomy for patients with esophageal cancer: a surveillance, epidemiology, and end-results study. Int J Radiat Oncol Biol Phys 2009;73:449-55. [Crossref] [PubMed]
- Malthaner RA, Wong RK, Rumble RB, et al. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med 2004;2:35. [Crossref] [PubMed]
- Burmeister BH. Role of radiotherapy in the pre-operative management of carcinoma of the esophagus. World J Gastrointest Oncol 2015;7:1-5. [Crossref] [PubMed]
- Arnott SJ, Duncan W, Gignoux M, et al. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev 2005.CD001799. [PubMed]
- Visser BC, Venook AP, Patti MG. Adjuvant and neoadjuvant therapy for esophageal cancer: a critical reappraisal. Surg Oncol 2003;12:1-7. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Gu Y, Swisher SG, Ajani JA, et al. The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer 2006;106:1017-25. [Crossref] [PubMed]
- Huang G, Gu XZ, Wang LJ, et al. Experience with combined preoperative irradiation and surgery for carcinoma of the esophagus. Gann Monogr Cancer Res 1986;31:159-64.
- Gignoux M, Roussel A, Palliot B, et al. The value of preoperative radiotherapy in esophageal cancer: results of a study of the EORTC. Recent Results Cancer Res 1988;110:1-13. [Crossref] [PubMed]
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049-53. [Crossref] [PubMed]
- Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol 2004;31:450-64. [Crossref] [PubMed]
- Younes M, Henson DE, Ertan A, et al. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol 2002;37:1359-65. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg 2003;75:331-6. [Crossref] [PubMed]




