Correlation of tumor mutational burden and treatment outcomes in patients with colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States and approximately 134,490 new cases will be diagnosed in 2016 alone (1). The Cancer Genome Atlas (TCGA) identified 16% of CRCs to have defects in mechanisms that repair spontaneous DNA damage, and consequently these tumors have increased mutational burden (2). While three quarters are due to mismatch repair defect (MMR-D) phenotype, otherwise known as high-frequency microsatellite instability (MSI-H) CRC, the remaining 25% consist of somatic mutations in one or more DNA repair genes such as POLE, BRAF, PIK3CA and PTEN (2,3).
Recent evidence showing improved responses to anti-PD-1 antibodies in MSI-H CRC has renewed the interest in its detection (4). Traditionally detected using immunohistochemistry or polymerase chain reaction (PCR), it is now possible to predict MSI-H CRC phenotype using mutational load extracted from next generation sequencing (NGS) (5,6). NGS can detect MSI-H CRC with concordance rates as high as 97% compared to standard testing (7).
Studies using MSI-H as a biomarker for chemotherapy response have yielded inconsistent results. A pivotal clinical trial using MSI-H as a biomarker showed that 5-fluorouracil (5-FU) adjuvant chemotherapy was not beneficial in decreasing colon cancer recurrence in MSI-H CRC (8). However, MSI-H tumors were shown to be sensitive to irinotecan chemotherapy with response in 57.1% (4 of 7) compared to only 10.8% (7 of 65) with microsatellite stable (MSS) CRC (P=0.009) (9). A trend toward longer DFS was also observed with the combination of irinotecan, 5-FU and leucovorin (LV) on MSI-H tumors as compared with those receiving 5-FU/LV (HR =0.57; 95% CI, 0.42–0.71 vs. HR =0.76; 95% CI, 0.64–0.88; P=0.07) (10). Another retrospective study of CRC patients treated with irinotecan based regimen in first line metastatic setting showed median progression-free survival (PFS) difference of 8.85 vs. 6.82 months in MSI-H and MSS respectively, but this difference did not reach statistical significance (P=0.089) (11).
CRC with unusually high mutational load (>150) may be attributed to a hypermutating phenotype such as the P286R hotspot POLE mutation (12). While such isolated mutations occur rarely, other DNA repair defects may cause tumors to have a higher mutation burden. Our recent study exploring the TCGA database showed that CRC cases carrying mutations in one of the 13 most frequently mutated DNA repair genes (ATM, MRCA2, MSH6, MLH1, LIG1, POLE, BRCA1, MSH2, SLX4, FANCM and FANCD2) exhibited higher mutational burden (13). While there is accumulating evidence suggesting that tumors with high mutational burden may respond better to checkpoint inhibition (4,14), the relationship between tumor mutational burden (TMB) status and response to chemotherapy is unknown. In this retrospective study, we investigated if TMB was associated with treatment response to chemotherapy administered to CRC patients. Specifically, the research project aimed to address the following questions: (I) Is there a difference in PFS between low TMB (TMB-L) and intermediate/high TMB (TMB-I/H) patients treated with chemotherapy in first line metastatic setting? (II) Do TMB-L and TMB-I/H patients respond differently to oxaliplatin and irinotecan? (III) Is there an association between time to recurrence and TMB status in stage 2 and 3 CRC patients who received perioperative oxaliplatin?
Methods
Patient identification
Following IRB approval, CRC patients treated at Northwestern University oncology clinics between June 1, 2013 through May 31, 2016 who had their tumors submitted for NGS with FoundationOne® (Foundation Medicine Inc., Cambridge, MA, USA) were identified. The most recent tumor samples available in the repository were sent for NGS during routine clinical care of the patients.
Procedures
Demographic (date of birth, sex, race, date of diagnosis) and clinical information (including imaging results and assessment of response to treatment, chemotherapy treatment history) were obtained by chart review. Treatment in the first-line metastatic setting was captured as ‘oxaliplatin-based’ if the chemotherapy regimen contained oxaliplatin (such as FOLFOX or XELOX) or ‘irinotecan-based’ such as FOLFIRI. No patients received oxaliplatin and irinotecan concomitantly. Dose modifications for oxaliplatin or irinotecan, or omission of the drug due to toxicity were not captured. Details of adjuvant chemotherapy received were noted. Patients were followed longitudinally until the progression of the cancer by periodic imaging (CT or MRI) as per the treating physician.
Extraction of DNA and NGS
At least 50 ng DNA was extracted from archival formalin-fixed, paraffin embedded tumor tissue and mutational data obtained by using hybridization capture of 3,769 exons of 315 cancer-related genes plus introns from 28 genes commonly rearranged in cancer (15,16). All classes of genomic alterations (base substitutions, small insertions and deletions (INDELS), rearrangements, copy number alterations) were determined. TMB was calculated by counting all synonymous and nonsynonymous variants as well as indels across a 1.25 megabase coding region spanning 315 genes. Germline polymorphisms were filtered using a proprietary somatic/germline algorithm (SGZ) and by comparing dbSNP, ExAC as well as internal FMI databases. Due to the high mutation rate associated with oncogenes and tumor suppressors, these genes were removed to limit biasing in the calculation of TMB (17). TMB-L and TMB-I/H were defined as ≤5 mutations per base (MB) or ≥6/MB respectively. MSI-status was detected using a validated novel computational method developed by Foundation Medicine Inc. (7).
Statistical analysis
Continuous variables were reported as medians and inter-quartile ranges and the two cohorts TMB-L (≤5/mbp) or TMB-I/H (≥6/mbp) compared between groups via the Wilcoxon rank-sum test. Categorical variables were reported as frequencies and percentages and compared between groups via Fisher’s exact test. PFS was defined as time from date of start of first line chemotherapy to the date of progression noted on imaging. Survival estimates were compared between TMB-L and TMB-I/H groups via the log-rank test. Hazard ratios were obtained via Cox regression. All analyses were conducted using SAS (SAS Institute Inc., Cary, NC, USA. Version 9.4).
Results
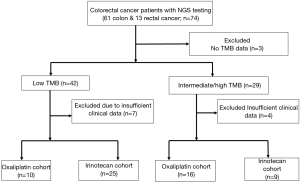
Seventy-four patients with CRC whose tumors were submitted to NGS testing were identified; 61 patients had colon cancer and 13 rectal cancer. Fourteen patients were excluded from analysis due to incomplete data (3 with no TMB data; 11 with insufficient clinical data) (Figure 1).
Impact of TMB status on PFS in metastatic CRC
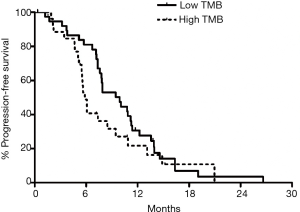
When outcomes of TMB-L (n=39) and TMB-I/H (n=26) were compared, without considering the nature of chemotherapy received in first line metastatic setting, a trend towards improved PFS was observed in the TMB-I/H compared to TMB-L (9.9 vs. 5.8 months, P=0.18) (Figure 2).
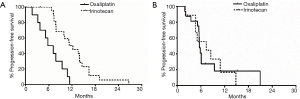
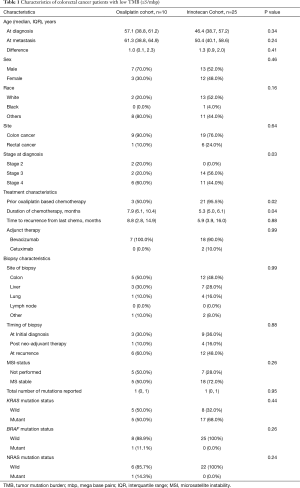
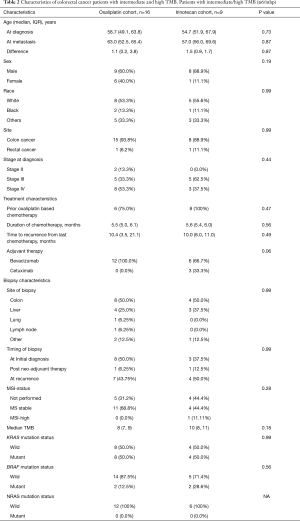
On subset analysis of TMB-L, patients in irinotecan cohort had improved PFS (11.7 months, n=25) compared to oxaliplatin (6.4 months, n=10), which was statistically significant (P=0.0002; HR =0.22; 95% CI, 0.09–0.52) (Figure 3A). However, oxaliplatin cohort had more stage 4 patients (60% vs. 44%) and a higher median age (57.1 vs. 46.4 years at initial diagnosis, P=0.34; 61.3 vs. 50.4 years at metastatic disease, P=0.24) compared to irinotecan cohort (44%) (Table 1). Majority of the patients in irinotecan cohort had received prior oxaliplatin-based therapy in adjuvant setting (95%). There was no significant difference in the molecular characteristics in terms of MSI-H status, median number of mutations, KRAS, NRAS and BRAF status between the two cohorts.
Full table
There was no difference in PFS between the irinotecan and oxaliplatin cohorts in TMB-I/H group, where the clinical and molecular characteristics were comparable between the two cohorts (Table 2) (Figure 3B).
Full table
TMB status and CRC recurrence after perioperative oxaliplatin based regimen
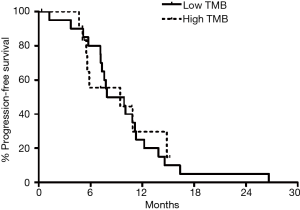
Twenty-nine patients had been diagnosed with stage 2 and 3 CRC and received perioperative chemotherapy. All patients were treated with oxaliplatin-based chemotherapy in perioperative setting. There was no difference in time to recurrence in the TMB-L and TMB-I/H groups in these patients (Figure 4).
Discussion
Our results suggest that a TMB obtained from NGS tumor profiling panel based on the numeric quantitation of the mutation load can serve as a predictive biomarker for tumor response to chemotherapy. Patients with TMB-L seemed to have improved PFS when treated with irinotecan-based chemotherapy compared to oxaliplatin.
Population level genetics show better prognosis in MSI-H phenotype, a tumor traditionally known to have high TMB (18,19). Patients with TMB-I/H in our study tended to have worse outcomes, with decreased PFS, although statistically not significant. This may partly be explained by sampling bias as NGS was obtained retrospectively in only those MSI-H tumor patients who progressed to metastatic CRC. This may inadvertently have selected the aggressive phenotype of otherwise less aggressive MSI-H phenotype.
Other limitations include, biopsies for NGS were obtained as warranted for the clinical care of the patients and the most recent biopsy available were sent, hence we cannot ensure uniformity in the timing of biopsy. The dose intensity received and concurrent administration of biologics was not captured. This is a small data set of patients from a single institution in USA and findings may not be generalizable. Our study did not analyze the origin of the cancer, which could have been of prognostic importance based on recent meta-analysis suggesting that left colon tumors were associated with reduced risk of death (HR =0.82; 95% CI, 0.79–0.84; P<0.001) (20). Further studies are needed corroborate these findings in a larger cohort, and we recommend investigating TMB on a tissue repository from clinical trials comparing irinotecan-based versus oxaliplatin-based regimens.
Acknowledgements
We would like to thank the Woman’s Board of Northwestern Memorial Hospital for their continued support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This study was approved by the Northwestern University Institutional review board of STU00200761. This approval exempted us from obtaining informed consent as this was a retrospective tissue study.
References
- SEER data. Accessed 10/6/2016. Available online: http://seer.cancer.gov/statfacts/html/colorect.html
- Muzny DM, Bainbridge MN, Chang K, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Lin EI, Tseng LH, Gocke CD, et al. Mutational profiling of colorectal cancers with microsatellite instability. Oncotarget 2015;6:42334-44. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69-77. [Crossref] [PubMed]
- Battaglin F, Stadler ZK, Cercek A, et al. Using mutational load in next generation sequencing (NGS) to identify mismatch repair (MMR) deficiency in colorectal cancer (CRC). ASCO Annual Meeting; Chicago, IL, USA. J Clin Oncol 2015;33:abstr 3565.
- Hall MJ, Gowen K, Sanford EM, et al. Evaluation of microsatellite instability (MSI) status in gastrointestinal (GI) tumor samples tested with comprehensive genomic profiling (CGP). 2016 Gastrointestinal Cancers Symposium; San Fransisco, CA, USA, 2016.
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57. [Crossref] [PubMed]
- Fallik D, Borrini F, Boige V, et al. Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res 2003;63:5738-44. [PubMed]
- Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol 2009;27:1814-21. [Crossref] [PubMed]
- Kim JE, Hong YS, Ryu MH, et al. Association between deficient mismatch repair system and efficacy to irinotecan-containing chemotherapy in metastatic colon cancer. Cancer Sci 2011;102:1706-11. [Crossref] [PubMed]
- Stadler ZK, Battaglin F, Middha S, et al. Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next-Generation Sequencing Panels. J Clin Oncol 2016;34:2141-7. [Crossref] [PubMed]
- Chae YK, Anker JF, Carneiro BA, et al. Genomic landscape of DNA repair genes in cancer. Oncotarget 2016;7:23312-21. [Crossref] [PubMed]
- Gargiulo P, Della Pepa C, Berardi S, et al. Tumor genotype and immune microenvironment in POLE-ultramutated and MSI-hypermutated Endometrial Cancers: New candidates for checkpoint blockade immunotherapy? Cancer Treat Rev 2016;48:61-8. [Crossref] [PubMed]
- Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023-31. [Crossref] [PubMed]
- Konduri K, Gallant JN, Chae YK, et al. EGFR Fusions as Novel Therapeutic Targets in Lung Cancer. Cancer Discov 2016;6:601-11. [Crossref] [PubMed]
- Johnson DB, Frampton GM, Rioth MJ, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res 2016;4:959-67. [Crossref] [PubMed]
- Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev 2001;10:917-23. [PubMed]
- Brueckl WM, Moesch C, Brabletz T, et al. Relationship between microsatellite instability, response and survival in palliative patients with colorectal cancer undergoing first-line chemotherapy. Anticancer Res 2003;23:1773-7. [PubMed]
- Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016. [Epub ahead of print]. [PubMed]


