Pancreatic non-functioning neuroendocrine tumor: a new entity genetically related to Lynch syndrome
Introduction
Lynch syndrome (LS), previously known as hereditary nonpolyposis colorectal cancer, is an autosomal dominant condition with incomplete penetrance. It is caused by a mutation in one of the deoxyribonucleic acid (DNA) mismatch repair (MMR) genes which leads to its loss of expression and function. Mutations occur most frequently in the MLH1 and MSH2 genes, followed by MSH6 and then finally PMS2 (1-3).
Colorectal carcinoma (CRC) is the neoplasm most commonly associated with LS. It is often diagnosed in young people under the age of 50 years, and accounts for 3–5% of all CRC (1,2). Endometrial carcinoma (EC) is the second most frequent malignancy in women. There are other less commonly associated neoplasms, like ductal adenocarcinomas of pancreas.
Pancreatic neuroendocrine tumors (P-NETs) are the second most common group of pancreatic tumors in the general population (4). Although most P-NETs are sporadic, some of them appear in the context of hereditary syndromes. The most commonly associated syndromes are multiple endocrine neoplastic disease type 1 (MEN1), Von Hippel-Lindau syndrome (VHL), neurofibromatosis type 1 (NF1) and tuberous sclerosis (TS).
Non-functioning pancreatic neuroendocrine tumors (NFP-NET) are the most frequent P-NETs. The coexistence of LS and P-NET has only rarely been described but the association between them has not been confirmed (3).
We report a patient with confirmed LS who developed many of the neoplasms included in the spectrum of this syndrome. We pay special attention to the presence of NFP-NET, in which we demonstrate the relationship between LS and P-NET by immunohistochemistry (IHC) (loss of expression of proteins MLH1 and its dimer PMS2) and the detection of microsatellite instability (MSI) using polymerase chain reaction (PCR).
Case presentation
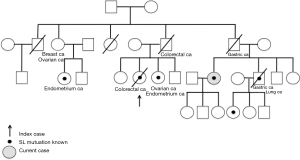
Our patient is a 65-year-old female from a family affected by LS. The index case was a paternal cousin of the patient. Family history is available in Figure 1.
At the age of 45, a regular screening colonoscopy for CRC (due to father’s death related to CRC) revealed left CRC. A sigmoidectomy was performed. The pathology study showed a pT1N0M0 adenocarcinoma. At age 57, she was diagnosed of a right CRC, and a right hemicolectomy was performed. Pathology study confirmed a pT1N0M0.
At age 58, she underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy for uterine cancer. Pathology study showed a pT1aN0M0 low grade endometrioid adenocarcinoma. Given the known family history with a cousin and a brother with LS, MMR protein IHC was performed, which revealed loss of expression of MLH1 and PMS2 proteins in tumor cells of endometrioid carcinoma and both CRC.
As the patient met the Amsterdam II and Bethesda criteria (Table 1), a genetic study was carried out which detected a MLH1 MMR gene mutation, c.731G > A (p.Gly244Asp), in accordance with the mutation found in the affected relatives. The patient’s sons also underwent genetic study but were not carriers of the mutation.
At age 63, a tumor in the right breast was detected on screening mammography and was treated with a lumpectomy. Histology study revealed an invasive low grade ductal carcinoma.
At age 65, a screening gastroscopy (due to the death of a brother with gastric cancer) revealed a duodenal adenocarcinoma. A pancreaticoduodenectomy with pylorus preservation operation was performed. The histology study reported three neoplasms: a pT1N0 duodenal low grade adenocarcinoma, and two incidental NFP-NET in the pancreatic head, one of 11 mm (G2, Ki67: 3%) and another of 7 mm (G1, Ki67: <1%).
A few months later, excision of a skin lesion was diagnosed as sebomatricoma.
IHC study was carried out on all the tumors presented, which confirmed the lack of nuclear expression of MLH1 and PMS2 proteins and the persistence of MSH2 and MSH6 expression in all but the G1 NFP-NET, in which the four proteins remained expressed (Figures 2,3).


MSI was also studied. MSI is generated at DNA level due to incorrect functioning of the DNA MMR system. We analyzed five microsatellites (repetitive DNA composed of sequences ranging in length from one to five DNA base-pairs) which are monomorphic and highly stable in the normal population. If the result shows two or more of them with extra DNA sequences, it is considered unstable (MSI-H) and is attributed to a dysfunction in DNA repair. If additional sequences are not found in any microsatellite, it is considered stable (MSI-S). Occasional cases show additional sequences in only one microsatellite, and it is considered as low unstable (MSI-L).
The study was conducted by PCR on patient samples, which showed MSI-H in colonic, duodenal and skin neoplasms, but were stable in the other samples (Figure 4).

Follow-up of patient showed no recurrences three years after the last surgery.
Discussion
This is the first case described in the literature that associates LS and NFP-NET. LS is caused by inactivating mutations of DNA MMR genes that impair DNA MMR mechanisms (1-3).
The diagnosis of LS is based on clinical suspicion (Table 1) and confirmed by germline genetic study of mutations in MMR genes (in order of frequency: MLH1, MSH2, MSH6 and PMS2) (1,2,5,6). LS is associated with CRC at an early age, and EC in female. Ovarian, gastric, urothelial, intestinal, pancreatic, hepatobiliary and breast carcinomas are also associated with LS, as well as skin and central nervous system tumors (1,2,6). Nowadays, families comprise fewer members than some decades ago, so there is less chance to diagnose LS based on clinical criteria.
IHC study of tumor specimens allows detection of the loss of expression of proteins encoded by MMR genes (1,3). In the case of an MLH1 gene mutation, loss of expression of the encoded protein prevents protein dimer formation with PMS2, which is recruited after MLH1. In our case, loss of expression of PMS2 is conditioned by the mutation detected in MLH1. This feature was observed by IHC in all the tumors analyzed with the exception of NFP-NET G1 (Figures 2,3).
MSI study of tumor samples was performed too (3). MSI-H was identified in colon, duodenum and skin neoplasms, but not in the others (Figure 4). This may have been because the microsatellites studied to detect MSI-H by PCR in our laboratory were more specific for CCR, so the absence of MSI-S detection in other malignancies does not rule out the diagnosis of LS. Thus, loss of protein expression, even in the case of a negative PCR result, is considered enough to link the tumor with LS. Moreover, the existence of MSI-H without loss of MMR protein expression is not diagnostic of LS (7). MSI is detected only in 35% of LS cases meeting Amsterdam II and Bethesda criteria (1).
Some data have associated LS and pancreatic tumors. There is a cumulative risk of pancreatic adenocarcinoma in LS patients of 1.31% (95% CI: 0.31–2.32%) at 50 years and 3.68% (95% CI: 4.7–15.7%) at 70 years compared to the general population (8). This association is included in the latest review of the Bethesda Guidelines (6).
Most pancreatic adenocarcinomas described as associated with LS reveal mutations in the MMR repair gene MSH2 (7,9,10), as often happens in extra-colonic tumors. Kastrinos et al. report that of the 47 cases of pancreatic adenocarcinomas, 31 had a mutation in the MSH2 gene, 13 cases in the MLH1 gene and three in the MSH6 gene (7). The most common pancreatic tumors associated with LS are ductal adenocarcinoma (7,10), although some cases of intraductal papillary mucinous neoplasm (IPMN) have been suggested (9).
P-NET is the second most frequent pancreatic tumors. The non-functioning type is the most prevalent (4). Clinical manifestations are usually silent or poor, often diagnosed incidentally or because of a mass effect. Although most P-NET is sporadic, some appear in the context of a hereditary syndrome, the most common are: MEN1 disease, VHL disease, NF1 and TS.
Karamurzin et al. described a case of NFP-NET in a patient with LS, but IHC examination of tumor did not show loss of expression of any MMR protein and there were no MSI-H (3). Therefore, the association between LS and P-NET has been suggested but has not been confirmed to date (3).
In our patient, a germline mutation of MLH1 was demonstrated in the biggest NFP-NET. But it was not demonstrated in the little one. Hypothesis for the retained protein expression in the small one is that complete loss of MMR function requires multiple cell cycle alterations and could be a late event in the neoplastic transformation (9).
Small intestine tumors account for fewer than 2% of gastrointestinal tract tumors in the general population (11). There are several inherited syndromes that increase the likelihood of developing this condition, such as familial adenomatous polyposis (FAP) disease, Peutz-Jeghers disease and LS (11,12). In LS, the probability of small intestine carcinoma ranges from 1–4%, around 100 times higher than in the general population (11,12). Adenocarcinomas are the most common type (as in our patient), with one reported case of carcinoid-type neuroendocrine tumor. Involvement of duodenum and jejunum is higher than terminal ileum. Attempts to determine whether the risk of small intestine carcinoma differs according to the MMR protein affected have shown it to be higher in cases of MLH1 and MSH2 mutations, with no difference between them (11). Incidence of small intestine carcinoma is so low that screening tests such as capsule endoscopy or gastroscopy are not recommended (13). Nevertheless, an increased detection of duodenal tumors has been observed with gastroscopy explorations performed as screening for the detection of gastric carcinoma (14). We consider that gastroduodenoscopy should be indicated in patients with LS as a screening test for gastroenteric neoplasms.
Conclusions
Our report supports the inclusion of P-NET in the list of extracolonic LS-associated tumors. P-NET must be included in the screening list as well as duodenal adenocarcinomas. We consider that in LS patients, a gastroduodenoscopy with endoscopic ultrasonography should be considered and an enhanced CT scan with an arterial and portal vein phase could help to discover P-NET tumors.
Acknowledgements
This case report was developed during 1 year. Special thanks to CM Blázquez Maña and R Carrera Salas for their support and the review of all the IHC and polymerase chain reaction results.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Steinke V, Engel C, Büttner R, et al. Hereditary Nonpolyposis Colorectal Cancer (HNPCC) / Lynch Syndrome. Dtsch Arztebl Int 2013;110:32-8. [PubMed]
- Barrow E, Hill J, Evans DG. Cancer risk in Lynch Syndrome. Fam Cancer 2013;12:229-40. [Crossref] [PubMed]
- Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair–deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol 2012;43:1677-87. [Crossref] [PubMed]
- Dickson PV, Behrman SW. Management of pancreatic neuroendocrine tumors. Surg Clin North Am 2013;93:675-91. [Crossref] [PubMed]
- Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. [Crossref] [PubMed]
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch Syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. [Crossref] [PubMed]
- Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch Syndrome. JAMA 2009;302:1790-5. [Crossref] [PubMed]
- Lindor NM, Petersen GM, Spurdle AB, et al. Pancreatic cancer and a novel MSH2 germline alteration. Pancreas 2011;40:1138-40. [Crossref] [PubMed]
- Flanagan MR, Jayaraj A, Xiong W, et al. Pancreatic intraductal papillary mucinous neoplasm in a patient with Lynch syndrome. World J Gastroenterol 2015;21:2820-5. [Crossref] [PubMed]
- Banville N, Geraghty R, Fox E, et al. Medullary carcinoma of the pancreas in a man with hereditary nonpolyposis colorectal cancer due to a mutation of the MSH2 mismatch repair gene. Hum Pathol 2006;37:1498-502. [Crossref] [PubMed]
- Park JG, Kim DW, Hong CW, et al. Germ line mutations of mismatch repair genes in hereditary nonpolyposis colorectal cancer patients with small bowel cancer: International Society for Gastrointestinal Hereditary Tumours Collaborative Study. Clin Cancer Res 2006;12:3389-93. [Crossref] [PubMed]
- Babba T, Schischmanoff O, Lagorfe C, et al. Small bowel carcinoma revealing HNPCC syndrome. Gastroenterol Clin Biol 2010;34:325-8. [Crossref] [PubMed]
- Koornstra JJ. Small bowel endoscopy in familial adenomatous polyposis and Lynch syndrome. Best Pract Res Clin Gastroenterol 2012;26:359-68. [Crossref] [PubMed]
- Yagyu T, Aihara T, Murayama M, et al. Mucinous carcinoma of the duodenum associated with hereditary nonpolyposis colorectal cancer: report of a case. Surg Today 2006;36:1129-32. [Crossref] [PubMed]



