Adjuvant chemotherapy and outcomes in esophageal carcinoma
Introduction
Esophageal cancer is the eighth most commonly diagnosed cancer and sixth leading cause of cancer-related deaths worldwide. In the year 2015, approximately 16,980 new cases of esophageal cancer were diagnosed in the US and 15,590 died from the disease (1,2). While the incidence of squamous cell carcinoma (the most common histological subtype worldwide) is decreasing in the West, adenocarcinoma is becoming increasingly prevalent in Northern Europe and in North America (3). Esophageal cancer has a high likelihood of metastasis as well as low 5-year survival rates (ranging from 15–25%). Although outcomes of patients with locally advanced disease have improved, their survival is still dismal in the majority of patients (4-6).
Multimodality therapy is currently the standard treatment for esophageal cancer based on the CROSS trial (7). While there have been several studies confirming the advantage of neoadjuvant chemoradiation on survival, there have been few on the benefit of postoperative chemotherapy for resected esophageal carcinoma (7-10). Pouliquen et al. examined the benefits of cisplatin and 5-FU, administered in 5-day courses every 28 days following surgical resection for esophageal squamous cell carcinoma (11). There was no significant difference in overall survival (OS) between the group that received adjuvant treatment and the group that underwent surgery alone (13 months in the untreated group and 14 in the treated group) even when they had incomplete (R1) resection. There was also no difference in quality of survival based on the duration of autonomous oral alimentation; however, the group that received treatment displayed greater hematologic, renal, and neurological toxicity (11). These patients, however, did not receive neoadjuvant therapy.
The role of postoperative chemotherapy remains unclear. We sought to review our large volume, single institution experience to determine the impact of such an approach in the setting of locally advanced disease.
Methods
Patients
Following IRB approval, the gastrointestinal (GI) department at Moffitt Cancer Center (MCC) established a database of esophagectomy cases by performing a retrospective chart review of patients operated on at MCC between June 1994 and March 2015. For this study, the database was queried according to our inclusion criteria: patients with esophageal carcinoma who underwent esophagectomy at our tertiary referral center from 1994–2015 following neoadjuvant chemoradiation (N=382). Of these patients, 46 received adjuvant therapy following surgery. Medical record information was obtained and recorded on standardized abstraction forms. Data collected included patient demographics, tumor characteristics, type of adjuvant therapy regimen, rationale for delivery of adjuvant therapy, and survival data. Patients were excluded who did not undergo surgical resection, did not receive neoadjuvant chemotherapy and radiation, or had known or suspected gross disease (either locoregional or metastatic) at the time adjuvant chemotherapy was delivered.
Statistical analysis
Analysis by 2-tailed Fisher’s exact test was used to determine whether adjuvant chemotherapy had any significant association with tumor location, histology, post-neoadjuvant therapy tumor classification (ypT), post-neoadjuvant therapy nodal classification (ypN), neoadjuvant chemotherapy regimen, pathologic response, margin status, sex, or ethnicity. Mann-Whitney U test (2-tailed) was used to assess correlation between adjuvant chemotherapy and patient age. Survival outcomes were analyzed using Kaplan-Meier method from date of diagnosis to the date of death or last follow up and compared by log-rank analysis. Confidence intervals (CI) represent 95% lower and upper bounds. Case-control group selection was performed using a 2:1 nearest neighbor propensity score matching algorithm (12). Covariates entered into the algorithm are those listed in Tables 1,2 with matching estimation by logistic regression. We performed all statistical analyses using SPSS (Windows Version 23.0; IBM Corp., Armonk, NY, USA). P must have been ≤0.05 to reach statistical significance.
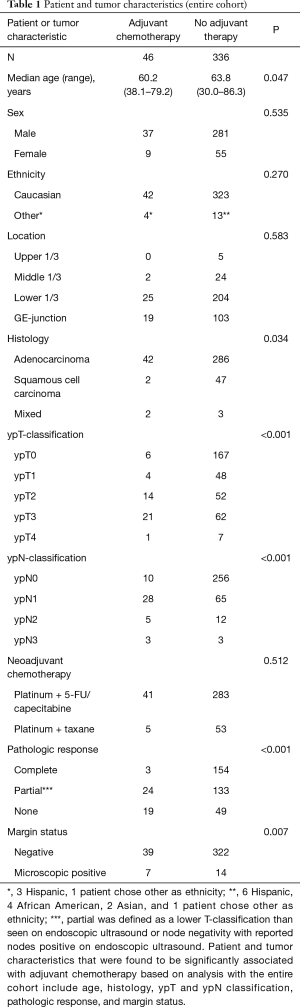
Full table
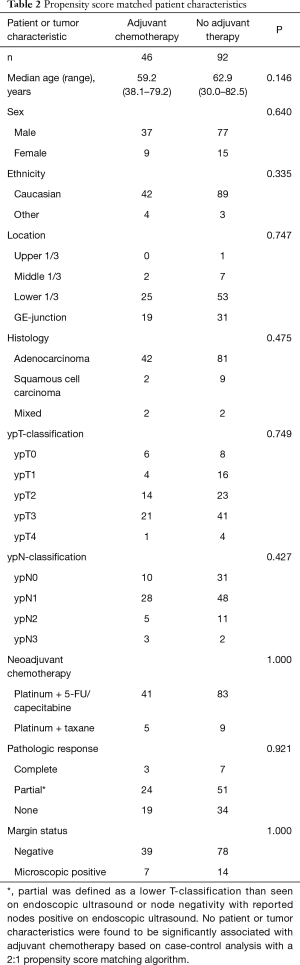
Full table
Results
Patient characteristics
Three hundred and eighty-two patients with esophageal carcinoma were identified for inclusion in this study; 46 of these patients received adjuvant therapy following surgery. No patients in this cohort received postoperative radiotherapy for their esophageal cancer. Patient characteristics before case-control matching are summarized in Table 1. Patients receiving adjuvant chemotherapy compared to no adjuvant chemotherapy were significantly younger (60.2 vs. 63.8 years; P=0.047), more likely to have adenocarcinoma (91% vs. 85%; P=0.034), more likely to have more advanced ypT or ypN classifications (<0.001 for both), more likely to have incomplete response to neoadjuvant therapy (93% vs. 60%; P<0.001), and more likely to have positive margins (15% vs. 4%; P=0.007).
Given the imbalances between these two groups, 2:1 nearest neighbor propensity score matching was performed to attempt to control for known confounding variables in an attempt to identify the effect of adjuvant chemotherapy on survival for patients treated with neoadjuvant chemoradiotherapy. The propensity score algorithm matched 46 patients in the adjuvant chemotherapy group to 92 patients in the no adjuvant chemotherapy group (n=126). Patient characteristics for this case-control analysis are summarized in Table 2. There were no significant differences in any patient or tumor characteristics after propensity score matching.
Adjuvant therapy
Of the 46 patients who were treated with postoperative chemotherapy, two patients received docetaxel alone, seven received 5-F/U and leucovorin, seven received 5-F/U and cisplatin, two received 5-F/U alone, four received carboplatin and paclitaxel, and two received carboplatin alone. There were eight patients for whom information on type of adjuvant chemotherapy was not available; the remainder received different combinations. We used notes in the medical records to search for the indications physicians used in deciding to recommend adjuvant therapy. Table 3 summarizes rationales for the delivery of postoperative treatment. The most common rationale for postoperative treatment was the presence of positive lymph nodes (N=36), and nine patients were found to have more than one reason for receiving adjuvant treatment. There were three patients for whom a rationale for postoperative chemotherapy was not clearly given.

Full table
Outcomes
OS and recurrence-free survival (RFS) were evaluated for the entire cohort as well as in case-control analysis. The median follow-up times for the entire cohort and for the case-control analysis were 2.9 and 2.4 years, respectively. There were no statistically significant differences in OS or RFS between the groups that did or did not receive adjuvant therapy in either of the analyses. Data for OS and RFS is summarized in Figures 1,2 for the entire cohort and for the propensity score matched cohort, respectively. For the entire cohort, the median OS time was 2.7 years (CI, 1.6–3.7) in the postop chemotherapy arm and 4.3 years (CI, 3.3–5.3) in the arm that did not receive postop chemotherapy (P=0.924). Median RFS were 2.2 years (CI, 1.8–2.6) and 3.3 years (CI, 2.3–4.3) for patients given adjuvant therapy and those who were not, respectively (P=0.874). In the case-control propensity-matching cohort, median OS was 2.7 years (CI, 1.6–3.7) in the group that received adjuvant chemotherapy and 2.8 years (CI, 2.0–3.6) in the group that did not receive any postoperative therapy (P=0.421). Median RFS was 2.2 years (CI, 1.4–3.0) and 2.2 years (CI, 1.8–2.9) for patients who underwent postoperative therapy and for patients who did not receive postoperative treatment, respectively (P=0.515).
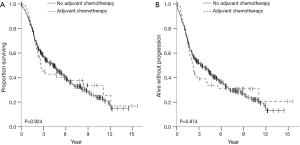
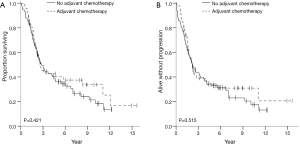
Discussion
Trimodality therapy is the standard treatment for esophageal cancer in the US. Data from the paradigm-establishing CROSS trial was associated with a 29% rate of pCR, 31% rate of pN+, 69% rate of pN0, and 92% rate of R0 resection compared with a 75% rate of pN+ and 26% pN0 for upfront resection (7). Prognostically, those patients who harbor residual disease post-neoadjuvant therapy have inferior outcomes, with data specifically identifying residual nodal disease as particularly ominous. Work from our group has added to this literature, reporting the survival implications of residual nodal disease in the setting of a pathologic CR at the primary site with a median OS difference of 92.2 vs. 14.8 months (P<0.01). In addition to residual nodal disease, other well-established risk factors for adverse outcomes include positive margin, angiolymphatic/perineural invasion, squamous cell histology, high preoperative alkaline phosphatase level, and poor performance status (13-17).
In an effort to improve outcomes, the role of adjuvant chemotherapy in patients treated with preoperative chemoradiation followed by surgery has been evaluated but remains unclear. There are conflicting results regarding whether postoperative chemotherapy in this setting confers any benefit. We found no survival benefit associated with adjuvant chemotherapy in our own analysis of patients treated with neoadjuvant chemoradiation followed by surgery for esophageal cancer. In a retrospective study of 145 patients who received trimodality therapy, Kim et al. found a survival advantage associated with adjuvant chemotherapy (18). The majority of patients were treated with a regimen of 5-fuorouracil and cisplatin and radiation to 50.4 Gy preoperatively, and 62 patients received chemotherapy following surgery, most with docetaxel. The 5-year OS and DFS were higher in patients who received postoperative chemotherapy and had macroscopic residual disease after neoadjuvant therapy: OS was 38.7% vs. 13.9% (P=0.016) and disease-specific survival was 42.8% vs. 18.8% (P=0.048). This benefit was not seen in those with pCR or those with microscopic residual disease (18). Tam et al. similarly found a benefit with postoperative chemotherapy in their retrospective cohort of 308 patients who received trimodality therapy for esophageal cancer; in patients who had a partial response and were treated with postoperative chemotherapy, there was a 26% decrease in relative hazard for long-term survival as compared to patients who received no further therapy following surgery (HR =0.74, 95% CI, 0.55–0.98) (19). These results are in contrast to those found by Yerramilli et al., who retrospectively compared clinical outcomes in 81 patients treated with or without chemotherapy following neoadjuvant chemoradiation and surgery for locally advanced esophageal cancer (20). Three-year OS and DFS were similar between patients who received adjuvant therapy to those who did not (74% vs. 70%; 60% vs. 64%; respectively). Interestingly, in patients who achieved pCR, adjuvant chemotherapy was associated with an improved 3-year OS (86% vs. 62%), but the difference did not reach statistical significance Similarly, in our analysis of 382 patients treated with trimodality therapy, of which 46 received adjuvant chemotherapy, there were no significant differences in either OS or progression free survival in the entire cohort and with propensity score-matched analysis. Given the conflicting results and lack of current consensus on adjuvant chemotherapy’s impact on survival outcomes in this setting, further randomized prospective trials are needed to clarify its role.
Given the poor outcomes associated with pathologic partial and non-responders, as well as patients with residual nodal disease, there is interest in evaluating whether adjuvant therapy can improve survival in this select group. The best pathologic outcomes in esophageal cancer—found in patients who achieve a pathologic complete response to neoadjuvant chemoradiation and have negative nodal status—are associated with the longest OS. We reported our survival outcomes based on nodal status in patients who had complete primary tumor response following trimodality therapy for esophageal cancer; the patients who had pathologic T0N0 had a median OS of 92.2 months, compared to only 14.8 months in patients who had pT0 but positive pathologic nodal status (P<0.001) (21). Donohoe et al. examined measures of pathologic response in patients with esophageal cancer treated with neoadjuvant therapy; the authors reported a survival of 71 months for pathologic complete responders compared to only 17 months in non/minimal responders (P<0.0001) (22). Some studies (discussed below) have stratified patients based on pathologic response, examining the role adjuvant chemotherapy plays in partial and non-responders compared to complete responders. Further studies must prospectively assess the role of adjuvant chemotherapy in those with pathologic non-response or positive pathologic nodal status to evaluate its role in settings of poor prognosis.
Some suggest that any benefits associated with adjuvant chemotherapy following trimodality treatment are potentially dependent on pathologic response to neoadjuvant chemoradiation. However, the evidence is minimal and inconsistent. Kim et al. found a significant 5-year OS and disease-specific survival benefit in patients with gross residual disease (no response to treatment) after neoadjuvant CRT (OS: 38.7% vs. 13.9%, P=0.016; CSS: 42.8% vs. 18.8%, P=0.048), while the same advantage was not found in patients with microscopic residual disease or a complete pathologic response (18). In Tam and colleagues’ analysis, a median survival benefit of 25.6 months (53.2 vs. 27.6 months) associated with adjuvant chemotherapy was found only in patients who achieved pathologic partial response to neoadjuvant CRT (P=0.047); in contrast, there was no difference in survival between those who received postoperative chemotherapy and those who did not among compete responders and non-responders (19). Yerramilli et al. only found a non-statistically significant improvement in 3-year OS with adjuvant chemotherapy in patients with a pathologic complete response (20). Such inconsistent data indicate the need for further prospective analysis to determine whether certain subgroups of patients who might potentially benefit from adjuvant chemotherapy can be identified based on pathologic response to neoadjuvant treatment.
There also remains the question as to whether adjuvant chemotherapy in this setting can be tolerated without excessive toxicity. In Yerramilli and colleagues’ analysis, the majority of patients treated with postoperative chemotherapy did not experience major toxicity, with the authors citing grade III/IV hematologic toxicity—including leukopenia, neutropenia, and thrombocytopenia—in only 11% of this group (20). The most commonly used postoperative chemotherapy regimens in this analysis were FOLFOX in 34% of patients, cisplatin/5-FU in 15%, 5-FU/LV in 15%, ECF in 13%, and carboplatin/paclitaxel in 9% (20). In a phase II study, Horgan et al. examined the tolerability and efficacy of postoperative chemotherapy with sunitinib in 61 patients who received surgery following neoadjuvant chemoradiation for esophageal and GE junction cancer (23). The authors found postoperative sunitinib to be poorly tolerated, with toxicity causing 33% of patients to discontinue its use. Additionally, the authors found no survival advantage associated with adjuvant chemotherapy (23).
Currently, the clinical rationales to assign adjuvant chemotherapy in this setting are wide-ranging and have yet to be clearly defined. Yerramilli et al. cited several rationales in their analysis; these included favorable pathologic response in 61% of cases, provider preference in 51%, and pathological nodal status in 32% (20). In our own analysis, the most common rationales for delivery of adjuvant chemotherapy were positive nodal status (78%) and positive resection margin (13%).
Both the role of adjuvant chemotherapy and the rationales for its administration in treating patients who receive neoadjuvant chemoradiation and surgery for esophageal cancer remain unclear. There are conflicting results regarding whether postoperative chemotherapy actually confers any survival advantage. Additionally, no widely accepted standard currently exists to guide the identification of patients who might benefit from postoperative chemotherapy after trimodality therapy. Prospective, randomized trials are needed to further elucidate the role of adjuvant chemotherapy for esophageal cancer in the context of trimodality treatment, particularly in the setting of the current emphasis on value-based care.
Conclusions
The role of chemotherapy following neoadjuvant chemoradiation and surgical resection in patients with locally advanced esophageal is unclear. In the largest series to date, our single institution retrospective review found no significant difference in overall or disease free survival in patients who received adjuvant chemotherapy status and those who did not. Future prospective studies should aim to further define the rationales for delivery of adjuvant chemotherapy, and to investigate any potential survival benefits conferred by adjuvant therapy. Several confounding factors might play a role in this retrospective study, including the fact that patients with complete response following neoadjuvant therapy and surgery have good overall outcome regardless of adjuvant therapy, and that most patients receive salvage therapy after recurrence even if they did not receive adjuvant therapy. Performance status following surgery might also play a role in determining whether physicians decide to treat patients with adjuvant therapy regardless of their pathological response and disease stage.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The study was approved by the ethics board of University of South Florida of IRB00000362. Patient personal information was not included and consent form was waived.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62:118-28. [Crossref] [PubMed]
- Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg 2009;87:1048-54; discussion 1054-5. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Shridhar R, Hayman T, Hoffe SE, et al. Body mass index and survival in esophageal adenocarcinoma treated with chemoradiotherapy followed by esophagectomy. J Gastrointest Surg 2012;16:1296-302. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89-96. [Crossref] [PubMed]
- Ikeda K, Ishida K, Sato N, et al. Chemoradiotherapy followed by surgery for thoracic esophageal cancer potentially or actually involving adjacent organs. Dis Esophagus 2001;14:197-201. [Crossref] [PubMed]
- Fréchette E, Buck DA, Kaplan BJ, et al. Esophageal cancer: outcomes of surgery, neoadjuvant chemotherapy, and three-dimension conformal radiotherapy. J Surg Oncol 2004;87:68-74. [Crossref] [PubMed]
- Pouliquen X, Levard H, Hay JM, et al. 5-Fluorouracil and cisplatin therapy after palliative surgical resection of squamous cell carcinoma of the esophagus. A multicenter randomized trial. French Associations for Surgical Research. Ann Surg 1996;223:127-33. [Crossref] [PubMed]
- Propensity Score Matching in SPSS: How to turn an Audit into a RCT. 2012. Available online: http://www.spssusers.co.uk/Events/2015/HAIR2015.pdf
- Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217-22; discussion 222. [Crossref] [PubMed]
- Siewert JR, Stein HJ, Feith M, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg 2001;234:360-7; discussion 368-9. [Crossref] [PubMed]
- Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278-88. [Crossref] [PubMed]
- Izbicki JR, Hosch SB, Pichlmeier U, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med 1997;337:1188-94. [Crossref] [PubMed]
- Liu L, Hofstetter WL, Rashid A, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol 2005;29:1079-85. [PubMed]
- Kim GJ, Koshy M, Hanlon AL, et al. The Benefit of Chemotherapy in Esophageal Cancer Patients With Residual Disease After Trimodality Therapy. Am J Clin Oncol 2016;39:136-41. [Crossref] [PubMed]
- Tam V, Hooker CM, Molena D, et al. Clinical response to neoadjuvant therapy to predict success of adjuvant chemotherapy for esophageal adenocarcinoma. J Clin Oncol 2014;32:137. [Crossref]
- Yerramilli D, Sohal D, Teitelbaum UR, et al. Adjuvant chemotherapy after trimodality therapy in locally advanced esophageal cancer. J Clin Oncol 2014;32:144. [Crossref]
- Blackham AU, Yue B, Almhanna K, et al. The prognostic value of residual nodal disease following neoadjuvant chemoradiation for esophageal cancer in patients with complete primary tumor response. J Surg Oncol 2015;112:597-602. [Crossref] [PubMed]
- Donohoe CL, O'Farrell NJ, Grant T, et al. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg 2013;258:784-92; discussion 792. [Crossref] [PubMed]
- Horgan AM, Darling G, Wong R, et al. Adjuvant sunitinib following chemoradiotherapy and surgery for locally advanced esophageal cancer: a phase II trial. Dis Esophagus 2016;29:1152-8. [Crossref] [PubMed]


