Probable paclitaxel-induced pancreatitis: uncommon case report and literature review
Introduction
Acute pancreatitis is an inflammatory disorder of the pancreas (1). Its diagnosis is based on the presence of at least two of the following three characteristics: abdominal pain consistent with acute pancreatitis, serum lipase or amylase levels that are at least 3 times the upper limit of the normal range, and findings of acute pancreatitis on cross-sectional imaging (2). Elevation of lipase levels is more specific and is thus preferred. In fact, serum amylase returns to normal values within 3–5 days after the onset of symptoms, in addition it may remain within the normal range on admission in as many as one-fifth of patients (3). Gallstones and alcohol are the two main etiologies (80%) (4).
Drug-induced pancreatitis is uncommon, its incidence varies between 0.1% to 2% (5). Drug-induced pancreatitis has been previously described with some antineoplastic agents such as l-asparaginase, 6-mercaptopurine, cisplatine, azathioprine, cytarabine and ifosfamide (6,7). Here we report an uncommon case of acute pancreatitis associated with paclitaxel therapy for ovarian cancer.
Case presentation
A 54-year-old female diagnosed with stage IC (FIGO) ovarian endometrioid adenocarcinoma. Decision of multidisciplinary meeting was to start with neoadjuvant chemotherapy with carboplatin (AUC6) and paclitaxel (175 mg/m2 over 3 h). Ten days after the first cycle, the patient was admitted to the hospital for severe epigastric pain. Physical examination showed epigastric tenderness, Laboratory analysis revealed elevated serum lipase to 1,141 IU/L (laboratory range 73–393 IU/L). Serum amylase test was not performed at admission; however it was performed on the fifth day and was normal. Serum LDH was elevated to 304 IU/L, calcium, triglycerides and serum IgG4 levels were normal. Other laboratory analysis were normal including liver function tests.
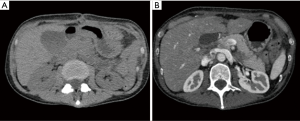
Abdominal ultrasound showed a normal liver and gall bladder, with no biliary ductal dilatation or gallstones. The pancreas was poorly visualized. A computed tomography (CT) scan showed diffuse pancreatic edema with infiltration of peripancreatic fat with no sign of necrosis. There was a hydrocholecyst; that was not observed on the abdominal ultrasound complementary exam; with no gallstones or biliary ductal dilatation (Figure 1). Computed tomography severity index (CTSI) was 2. Diagnosis of mild acute pancreatitis was made based on elevated lipase, clinical and imaging findings. The patient was treated with analgesics, proton pump inhibitors and intravenous hydration. Her pain resolved and serum lipase level was 773 IU/L after 2 days and gradually normalized within 2 months. A bili MRI was performed 12 days after the acute phase, it showed a total regression of the signs of acute pancreatitis without suspected pancreatic lesion or lithiasis of the bile ducts or biliary vesicles. The patient had no risk factors for acute pancreatitis, she had no history of alcohol consumption, she was not obese, triglycerides and serum IgG4 levels were normal and she did not take any other medications at home that may be suspected. Paclitaxel induced pancreatitis was suspected and it has not been re-administered. The patient received later five cycles of carboplatin without any complications.
Discussion
Paclitaxel-induced acute pancreatitis has been described only in seven cases (Table 1). The time to onset of symptoms varied from one to thirty days after administration of paclitaxel and after one to five cycles. The paclitaxel dose administered for all patients was 175 mg/m2, except in one case where 80 mg/m2 dose was used. Pancreatitis was mild and was resolved after 1 to 9 days. One patient had a necrotic pancreatitis that required a pancreatic necrostomy and hospitalisation in the intensive care unit for three months due to multiples complications. In the seven others cases report, paclitaxel rechallenge was attempted in three of them and a positive rechallenge occur in two cases (6,8-13).
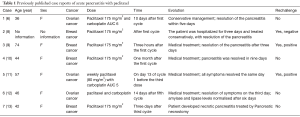
Full table
Our patient had presented a mild acute pancreatitis ten days after the first cycle. Clinical symptoms and imaging normalized rapidly; however, serum lipase level has been gradually normalized two months later. Diagnosis of drug-induced pancreatitis was suspected after exclusion of all common etiologies of acute pancreatitis such as alcohol, gallstones, hypercalcemia and hypertriglyceridemia. In addition, our patient did not take any other medications at home that may be suspected.
Carboplatin induced acute pancreatitis has been reported only in one case. Patient developed acute pancreatitis after the third and fourth cycles of adjuvant chemotherapy regimen including docetaxel, carboplatin, and trastuzumab for breast cancer. However, authors did not conclude whether the patient developed acute pancreatitis with either carboplatin or docetaxel, or with the combination of both (4). Our patient received carboplatin in the following cycles without any complications. Due to the frequent administration of concomitant agents, a causal relationship for drug-induced pancreatitis is particularly difficult to bear. Patients are often premedicated prior to chemotherapy. Our patient received premedication for paclitaxel hypersensitivity reaction including prednisolone (130 mg orally), ranitidine (50 mg IV), dexchlorpheniramine (5 mg IV), as well as setron (ondansetron 8 mg IV) to prevent nausea and vomiting. Ondansetron has been suggested as a cause of pancreatitis in a patient after prolonged use (14). Corticosteroids have been implicated in drug-induced pancreatitis (15). Nevertheless, our patient received these drugs in the following cycles without any complication. This decreases the likelihood that these drugs will be responsible for the reaction. No cases of acute pancreatitis were reported with dexchlorpheniramine (7,15). In the case of ranitidine, a record-linkage case-control study has objectified a crude significant association between cimetidine, ranitidine and acute pancreatitis, although this association disappeared after adjustment for potential confounders. A retrospective cohort study in the General Practice Research Database (GPRD) in the United Kingdom (UK) was made to evaluate the risk of acute pancreatitis associated with the use of acid-suppressing drugs including, ranitidine. The study concluded that there is no association between acute pancreatitis and the use of acid-suppressing drugs, although a substantial increase in risk cannot be excluded with confidence (16).
The mechanism of paclitaxel-associated pancreatitis is unknown. In animal studies, paclitaxel prevented cerulein-induced pancreatitis. However, this protective effect disappears when cremophor EL (CrEL) is used as a vehicle (6). CrEL is a formulation vehicle used for various poorly-water soluble drugs, including the anticancer agent paclitaxel (17). Paclitaxel is a hydrophobic molecule that is solubilized with CrEL to be administered to patients. Cyclosporine is also solubilized with CrEl, and several studies suggest that CrEl is implicated in cyclosporine-associated pancreatitis (10). Further research is needed to explain the mechanism by which paclitaxel can cause pancreatitis.
Conclusions
Because paclitaxel is used in many chemotherapy protocols, it is important for clinicians to be aware that paclitaxel can induce acute pancreatitis, as early diagnosis can be vital.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015;386:85-96. [Crossref] [PubMed]
- Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med 2016;375:1972-81. [Crossref] [PubMed]
- Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400-15; 1416.
- Singh V, Devata S, Cheng YC. Carboplatin and docetaxel-induced acute pancreatitis: brief report. Int J Clin Oncol 2010;15:642-4. [Crossref] [PubMed]
- Butt W, Saadati H, Saif MW. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res 2010;30:5113-5. [PubMed]
- Kumar DM, Sundar S, Vasanthan S. A case of paclitaxel-induced pancreatitis. Clin Oncol (R Coll Radiol) 2003;15:35. [Crossref] [PubMed]
- Nitsche C, Maertin S, Scheiber J, et al. Drug-induced pancreatitis. Curr Gastroenterol Rep 2012;14:131-8. [Crossref] [PubMed]
- Hudis C, Riccio L, Holmes F, et al. Phase II study of semisynthetic paclitaxel in metastatic breast cancer. Eur J Cancer 1997;33:2198-202. [Crossref] [PubMed]
- Hoff PM, Valero V, Holmes FA, et al. Paclitaxel-induced pancreatitis: a case report. J Natl Cancer Inst 1997;89:91-3. [Crossref] [PubMed]
- Mills KM, Johnson DM, Middlebrooks M, et al. Possible drug-associated pancreatitis after paclitaxel-cremophor administration. Pharmacotherapy 2000;20:95-7. [Crossref] [PubMed]
- Adam JP, Gauthier P, Letarte N. Safe administration of docetaxel after weekly paclitaxel-induced acute pancreatitis. J Oncol Pharm Pract 2017;23:540-4. [PubMed]
- Shintani D, Yoshida H, Imai Y, et al. Acute pancreatitis induced by paclitaxel and carboplatin therapy in an ovarian cancer patient. Eur J Gynaecol Oncol 2016;37:286-7. [PubMed]
- McMahon MA, Kearns G, McCaffrey J, et al. Association between paclitaxel and necrotic pancreatitis. Ir Med J 2006;99:281. [PubMed]
- Alberti-Flor JJ. Pancreatitis associated with ondansetron. J Natl Cancer Inst 1995;87:689-90. [Crossref] [PubMed]
- Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Ochsner J 2015;15:45-51. [PubMed]
- Eland IA, Alvarez CH, Stricker BH, et al. The risk of acute pancreatitis associated with acid-suppressing drugs. Br J Clin Pharmacol 2000;49:473-8. [Crossref] [PubMed]
- Gelderblom H, Verweij J, Nooter K, et al. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001;37:1590-8. [Crossref] [PubMed]


