Heated intraperitoneal chemotherapy and gastrectomy for gastric cancer in the U.S.: the time is now
Introduction
In the United States this year there will be an estimated 28,000 new cases and 10,960 deaths due to gastric adenocarcinoma (1). Although the current treatment paradigm of surgical resection and systemic therapy aims to increase recurrence-free and overall survival, peritoneal carcinomatosis remains a source of major morbidity and a frequent cause of death (2). Nearly 40% of patients with gastric adenocarcinoma will develop peritoneal metastasis during the course of their disease, and approximately 70% of these patients will have disease largely confined to the peritoneum (3). Peritoneal dissemination begins early, and is often unrecognized prior to development of overt peritoneal metastasis. Based on retrospective studies, positive peritoneal cytology is present in 7–13% of patients at time of curative surgery (4,5). Patients with positive peritoneal cytology experience a median survival of 12 months, which is similar to patients with macroscopic peritoneal metastasis.
Current data suggest an improvement in survival associated with intra-operative intraperitoneal (IP) chemotherapy for properly selected patients with carcinomatosis of gastric origin. Yang and colleagues conducted a prospective phase III randomized trial to evaluate IP chemotherapy with cytoreductive surgery compared to cytoreductive surgery alone for the treatment of gastric cancer with peritoneal carcinomatosis (6). The authors reported significantly improved median overall survival of 11.9 months with surgery plus IP chemotherapy compared to 6.5 months with surgery alone. Our own prospective randomized study of cytoreductive surgery, gastrectomy and heated IP chemotherapy (HIPEC) with or without systemic chemotherapy in patients with metastatic gastric cancer was reported by Rudloff et al. (7). Although underpowered, the study demonstrated that patients with peritoneal carcinomatosis and limited disease burden could achieve prolonged survival with cytoreduction, including gastrectomy, and IP chemotherapy. In most studies of IP chemotherapy like these, the key factors associated with improved survival include small disease burden [i.e., low peritoneal cancer index (PCI)] and ability to achieve complete cytoreduction (CC-0).
A clear benefit of gastrectomy in the presence of peritoneal disease is lacking despite an association with improved survival (8,9). The REGATTA trial remains the only prospective, randomized trial performed to evaluate the role of palliative gastrectomy (i.e., non-curative, without metastasectomy) prior to systemic therapy for patients with limited (solitary site) gastric cancer metastasis (10). Patients with gastric cancer and a single-site of metastasis, such as liver, para-aortic lymph nodes, or peritoneum, were randomly assigned to chemotherapy alone or chemotherapy with gastrectomy. Notably, the most common non-curable factor was peritoneal metastasis. No difference in survival between treatment arms was demonstrated, however, the study design did not allow for metastasectomy. In other words, the impact of resection of the primary tumor with aggressive control of metastatic disease was not evaluated.
Ishigami and colleagues recently evaluated the safety and efficacy of gastrectomy after response to systemic and IP chemotherapy in patients with gastric carcinomatosis (11). Gastrectomy (without peritonectomy or intraoperative IP chemo) was considered in 100 patients who achieved a cytopathologic, clinical and/or radiographic response to systemic and IP chemotherapy. The median overall survival of the 64 patients selected for surgery was 30.5 months from the initiation of IP chemotherapy. Factors associated with extended survival included selection for surgery included low volume peritoneal disease (P0/cytology-positive or P1) and histologic response to therapy (viable tumor in ≤1/3 of tumor area). Identification of early peritoneal metastasis may therefore reveal a group of patients in whom regional therapy can be rationally applied to interrupt the peritoneal metastatic cascade and thereby extend survival.
Based on these data, it is anticipated that patients with isolated peritoneal disease and the lowest burden of metastasis will reap the greatest benefit from a strategy that includes regional therapy. We hypothesize that gastrectomy combined with HIPEC will improve survival in patients with gastric cancer associated with positive peritoneal cytology and/or very low-volume peritoneal carcinomatosis. Therefore, we have opened a phase II clinical trial for patients with gastric cancer and present the rationale and details for this study below.
Methods
A trial of HIPEC and gastrectomy for gastric cancer with positive peritoneal cytology was approved by the Institutional Review Board of the National Cancer Institute, National Institutes of Health (NIH), Bethesda, Maryland, USA (NCT03092518).
Design
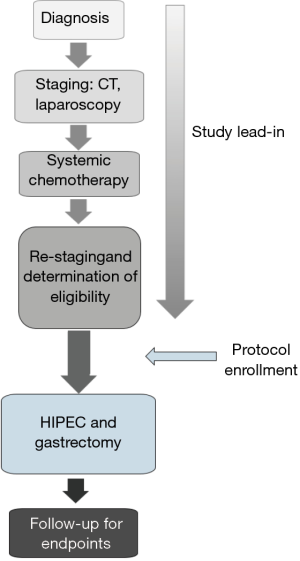
This is a single institution, phase II study designed to determine the efficacy of HIPEC and gastrectomy in patients with gastric cancer and associated positive peritoneal cytology or limited peritoneal carcinomatosis. Eligible patients are being enrolled on study and treated at the NIH Clinical Center (Figure 1). The opportunity exists to expand participation in the current study to other institutions.
Eligibility
Eligible patients must be greater than 18 years old with a performance status (ECOG) <2 and be physiologically able to undergo HIPEC and gastrectomy. Patients require histologically or cytologically confirmed gastric or gastroesophageal junction adenocarcinoma with subradiographic and/or cytopathologic evidence of peritoneal carcinomatosis found at staging laparoscopy. In addition, patients must have limited peritoneal carcinomatosis (≤ P1 or PCI <10) found at laparoscopy or on final pathology that is deemed completely resectable. Prior to definitive surgery, patient must have received minimum of 3 months of fluoropyrimidine and/or platinum based systemic chemotherapy.
Exclusion
Patients with disseminated extra-peritoneal or solid organ metastases, which includes carcinomatosis associated with clinically or radiographically evident ascites (greater than 500 mL) are excluded from the study. However, patients with greater omentum and ovarian metastases are accepted.
Intervention
Staging radiographic studies (CT and/or PET/CT) and laparoscopy are performed to rule out distant metastases prior to enrollment. Patients with distant or diffuse peritoneal carcinomatosis (≥ P2 or PCI >10) will not undergo treatment on study. These inclusion criteria are based on previously published data indicating that selection of patients with low-volume peritoneal disease are likely to experience improved survival compared to those with larger disease burden (12). Patients who meet eligibility will receive treatment with HIPEC and gastrectomy within 8 weeks of last dose of systemic chemotherapy. A lead-in of systemic therapy prior to HIPEC was selected to provide opportunity to assess pathologic tumor response to systemic chemotherapy, and allow a period to assess disease control.
At operation, patients undergo resection of the primary tumor based on location along with standard lymphadenectomy. All visible peritoneal disease is resected via peritonectomy of involved surfaces. Omental implants are removed as part of omentectomy and ovarian metastases, if present, are removed by oophorectomy. IP chemotherapy with cisplatin (90 mg/m2) and mitomycin C (10 mg/m2) is delivered via closed perfusion circuit using the Belmont hyperthermia pump (Billerica, MA, USA). The choice of cisplatin and mitomycin C is based on a systematic review of existing literature on IP chemotherapy for gastric cancer (13). The target IP temperature is set at 41 degrees Celsius with a flow rate of 1 L/min for 60 minutes. Sodium thiosulfate is infused intravenously starting with IP cisplatin administration and continues for a total of 12 hours thereafter. Manual manipulation of the abdomen accompanies peritoneal perfusion. After perfusion, the circuit and abdomen are flushed with saline. Gastrointestinal continuity is re-established and the operation concluded.
Post-operative care and follow-up
Patients receive standard post-operative monitoring with an expected hospitalization of 7 to 14 days. Patients discharged within this time frame are able to tolerate an oral diet with or without dietary supplements. Feeding jejunostomy tubes are not used routinely. Patients are evaluated for disease recurrence every 3 months starting at 3 months post-operatively for 2 years, and then semi-annually for 3 years thereafter. Evaluation consists of physical examination, nutritional assessment, general laboratory studies, CT chest/abdomen/pelvis, PET scan as needed, and quality of life questionnaire. Post-operative systemic therapy is administered at the discretion of the treating physician and is based on pathologic response to pre-operative therapy and patient performance status.
Study endpoints and correlative studies
The primary endpoint is overall survival as measured from the initiation of treatment with systemic chemotherapy. Secondary endpoints include determination of IP progression-free survival, distant (extra-peritoneal) disease-free survival, and evaluation of treatment-related morbidity. Additional exploratory endpoints and correlative studies are included to support our basic and translational research efforts. The proposed sub-classifications of gastric adenocarcinoma suggest that the phenotype of isolated peritoneal metastasis seen most often in poorly differentiated, diffuse type cancers is due to genomic alterations in cell-cell adhesion, cytoskeletal integrity and cellular motility pathways (14,15). Blood, tumor and tissue collected before, during and after treatment will support the ongoing study of the molecular drivers of peritoneal metastasis.
Statistical considerations
The median overall survival for patients with positive peritoneal cytology, or limited peritoneal carcinomatosis, receiving systemic chemotherapy alone is approximately 14 months (16,17). The primary aim of the study is to determine if systemic chemotherapy followed by HIPEC and gastrectomy could be associated with a 24-month median overall survival compared to these historical controls. The target accrual of 40 patients over 4 years provides 80% power to detect a 10-month improvement in medial overall survival (compared to historical controls), with a one-sided 0.10 alpha level test.
Conclusions
Prospective evaluation of HIPEC for gastric adenocarcinoma is overdue in North America. We believe the available data indicate therapeutic efficacy for the application of regional therapy in those patients likely to suffer abbreviated survival secondary to peritoneal metastasis. Further studies of this treatment strategy will require multi-institutional collaboration, standardization and more robust translational research. Therefore, we strongly encourage candidate patients be referred for evaluation and treatment on a registered clinical trial.
Acknowledgements
This study was supported by Intramural Research Program of the National Cancer Institute (NIH).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Sarela AI, Miner TJ, Karpeh MS, et al. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg 2006;243:189-95. [Crossref] [PubMed]
- D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004;240:808-16. [Crossref] [PubMed]
- Bentrem D, Wilton A, Mazumdar M, et al. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol 2005;12:347-53. [Crossref] [PubMed]
- Ikoma N, Blum M, Chiang YJ, et al. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Ann Surg Oncol 2016;23:4332-7. [Crossref] [PubMed]
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [Crossref] [PubMed]
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. [Crossref] [PubMed]
- Hartgrink HH, Putter H, Klein Kranenbarg E, et al. Value of palliative resection in gastric cancer. Br J Surg 2002;89:1438-43. [Crossref] [PubMed]
- Monson JR, Donohue JH, McIlrath DC, et al. Total gastrectomy for advanced cancer. A worthwhile palliative procedure. Cancer 1991;68:1863-8. [Crossref] [PubMed]
- Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016;17:309-18. [Crossref] [PubMed]
- Ishigami H, Yamaguchi H, Yamashita H, et al. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer 2017;20:128-34. [Crossref] [PubMed]
- Yonemura Y, Canbay E, Li Y, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol 2016;42:1123-31. [Crossref] [PubMed]
- Feingold PL, Kwong ML, Davis JL, et al. Adjuvant intraperitoneal chemotherapy for the treatment of gastric cancer at risk for peritoneal carcinomatosis: A systematic review. J Surg Oncol 2017;115:192-201. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol 2008;15:2684-91. [Crossref] [PubMed]
- Mezhir JJ, Shah MA, Jacks LM, et al. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol 2010;17:3173-80. [Crossref] [PubMed]