Complete pathologic response is independent of the timing of esophagectomy following neoadjuvant chemoradiation for esophageal cancer
Introduction
In 2015, 16,980 new cases of esophageal cancer were diagnosed in the United States, and about 15,590 patients died from this disease (1). The 5-year survival rate for all patients between 2004 and 2010 was only 20% (1). The epidemiology of this cancer has changed significantly in the last few years in the United States. Until about 30–40 years ago, squamous cell carcinoma (SCC) was more common than adenocarcinoma and comprised 90–95% of all cases of esophageal cancer (2). However, adenocarcinoma has increased steadily and now comprises more than 70% of new cases of esophageal cancer.
After diagnosis, the treatment for esophageal cancer typically involves a multi-disciplinary approach using a combination of surgery, chemotherapy and/or radiation, as dictated by the stage of disease presentation (3). While esophagectomy as the first line modality is reserved for select early stage cases, patients with T2 or higher disease have a significantly higher risk for lymph node metastasis and therefore are routinely treated with neoadjuvant chemoradiation (nCRT) (4-9). In patients treated with nCRT, the traditional approach is to perform esophagectomy within 4–8 weeks of completion of the treatment (10-12). Generally, it is believed that patients are not fully recovered from nCRT within 4 weeks of treatment and require time to recover before surgery is planned. On the other hand, delaying surgery beyond 8 weeks is believed to be associated with a higher rate of developing complications secondary to difficult dissection related to fibrosis after radiation (10,12). There do not exist studies that have specifically addressed the issue of timing of esophagectomy after nCRT. Thus, the optimal timing of esophagectomy after completing nCRT is unknown.
Similar to esophageal cancer, many patients with rectal cancer are treated with nCRT before surgical resection. However, unlike esophageal cancer, there are studies that support improvement in overall survival (OS) by delaying surgery by more than 7 weeks after neoadjuvant therapy (13,14). Using this information, similar studies have been performed in esophageal cancer. However, the major studies published thus far have revealed conflicting results. Although an early study by Ruol et al. showed a reduction in the incidence of recurrence by delaying esophagectomy (15), subsequent studies produced conflicting data on the relationship between the timing of esophagectomy with complete pathologic response (cPR) or survival (16).
Based on our observation that many patients are operated beyond the ‘established’ time frame of 4–8 weeks due to a variety of reasons, including insufficient recovery after nCRT, cardiac or pulmonary optimization, and patient or hospital scheduling logistics, we sought to analyze our institutional data focusing on the relationship between cPR and the timing of esophagectomy after nCRT. As we know from our data and others, cPR is associated with improved overall and disease specific survival (17). Thus, we hypothesized that delaying esophagectomy after nCRT results in increased cPR and thus may have an effect on OS.
Methods
All patients who underwent esophagectomy after completing nCRT for esophageal cancer between 2004 and 2014 were identified from a retrospective review of a prospectively maintained database at Roswell Park Cancer Institute. All patients had biopsy confirmed esophageal tumors. Patients who underwent salvage esophagectomy or were lost to follow up were excluded. All pertinent demographic and clinical data including age, gender, ECOG performance status, timing of surgery, post-operative length of stay and complications were collected. Tumor characteristics including location, histology, signet cells, differentiation, lymphovascular invasion, margin status, lymph node status, and cPR were recorded. This study received appropriate approval from the Institutional Review Board at Roswell Park Cancer Institute.
The timing of esophagectomy was determined as the time difference in months from the last date of chemotherapy or radiation to esophagectomy. cPR was determined based on the absence of any residual cancer on pathology, including both microscopic and macroscopic disease. Tumor locations were recorded as proximal third, middle third or distal third. Negative surgical margin (R0) was defined as macro- and microscopic absence of cancer cells at all resection margins on permanent section.
Patients were divided into two groups based on the timing of esophagectomy, i.e., early (≤50 days) and delayed (>50 days). The primary end-point of this study was OS. Patients were evaluated from the date of their first treatment (nCRT) to either the date of last clinical follow-up or death. Date of death was determined from the medical records and when not available from social security death index (SSDI). Patients who were alive at the date of last contact were censored at that date.
Statistical analysis
Patient demographics, clinical variables and treatment characteristics were analyzed as groups using the mean, median, standard deviation and ranges. Frequencies and relative frequencies were utilized for categorical variables. Comparisons were made between groups using the Mann-Whitney U and Fisher’s exact tests. Univariate logistic regression was utilized to identify factors that affected pathological response and timing of esophagectomy. Cox regression was used to evaluate timing of esophagectomy as a continuous variable and its association with cPR. To assess factors significantly affecting survival, Kaplan-Meier models were created. Log-rank test was used to evaluate the significant difference. Multivariable Cox proportional hazard models were used to evaluate whether any of these factors were independent predictors of overall or progression free survival (PFS). The results were expressed as odds and hazard ratio estimates with 95% confidence intervals, and P values were considered significant if ≤0.05. The models were fit using Firth’s penalized function and hazard ratios, with corresponding confidence intervals, and obtained from the model estimates. All statistical analyses performed were two-sided and generated using SAS v9.4 (Cary, NC, USA).
Results
Patient characteristics
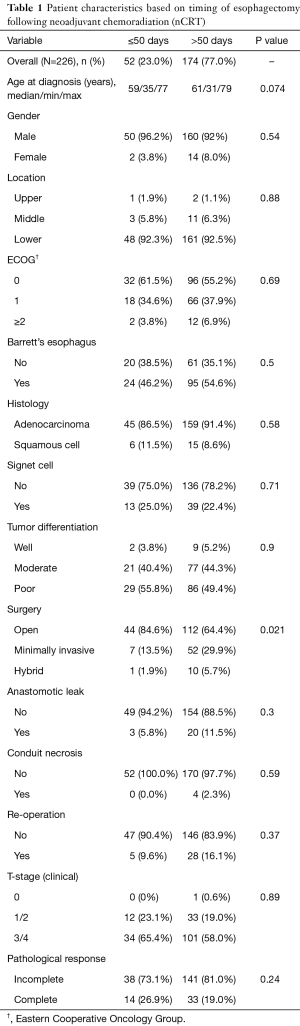
During the study period between 2004 and 2014, a total of 236 patients were available for analyses. Of these, 10 patients were excluded from the analyses due to inadequate post-treatment follow-up (n=5), incomplete documentation (n=3), or salvage esophagectomy (n=2). Thus, a total of 226 patients were available for the final analysis. Patient demographics and clinical characteristics based on timing of esophagectomy are shown in Table 1. There were 210 males (92.9%) and 16 females (7.1%). The median age was 61 years (range, 31–79 years), and the median follow-up period was 52 months (range, 2–110 months). Barrett’s esophagus was found in 119 patients (59.5%) among 200 patients in whom this data was available. Adenocarcinoma was diagnosed in 205 (90.7%) patients, and SCC in 21 (9.3%) patients. The tumors were well differentiated in 11 (4.9%), moderately differentiated in 98 (43.4%), poorly differentiated in 115 (50.9%) and undifferentiated in 2 (0.9%), respectively. Signet cell features were seen in 52 (23.0%) patients.
Full table
All patients were treated with a concurrent neoadjuvant protocol prior to resection. While the dose of radiation delivered remained generally similar throughout the 10-year period, the chemotherapy regimens varied. With regards to radiation, 217 (96%) patients received 50.4 Gy radiation, and 9 (4%) patients were treated with doses ranging from 45–48.9 Gy. Eighty-eight (38.9%) patients received a combination of cisplatin and irinotecan, 77 (34.1%) carboplatin and taxol, 48 (21.2%) oxaliplatin and capecitabine, 10 (4.4%) 5-fluorocil and cisplatin, and 3 (1.3%) patients were treated with other protocols.
Traditional open esophagectomy was performed in 156 (69%), minimally invasive esophagectomy in 59 (26%), and hybrid esophagectomy in 11 (4.9%) patients. There were no mortalities at 30 days, and the overall complication rate was 41.6% (n=94). The most common complication was pulmonary (including atelectasis, pneumothorax and pneumonia, n=63, 27.9%), and other complications included cardiac (arrhythmia and myocardial infarction, n=40, 17.7%), anastomotic leak (n=23, 10%), and conduit necrosis (n=5, 2.2%).
Timing of esophagectomy and relationship with cPR and survival
Fifty-two patients in early group (≤50 days) were compared to 174 patients in the delayed group. Patients in the early group (≤50 days) had a higher percentage of cPR, 27% versus 19%. However, this finding was not statistically significant (P=0.24). On analyzing OS and PFS based on timing of esophagectomy (Figure 1), the timing of esophagectomy was not associated with either survival outcome.
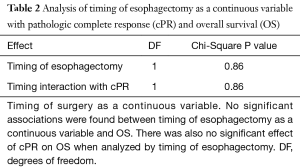
Next, we analyzed the association between survival outcomes (OS and PFS) with the timing of esophagectomy as a continuous variable, and its interaction with cPR (Table 2). Using a Cox regression model, we found that for both OS and PFS there was a statistically significant association between survival and cPR status, as expected. However, there were no significant associations between OS or PFS and the timing of esophagectomy as a continuous variable. Further, there was no statistically significant interaction noted between cPR, the timing of esophagectomy, and OS or PFS. In other words, the association of cPR with OS and PFS was similar regardless of when esophagectomy was performed.
Full table
Multivariable analysis of factors associated with survival
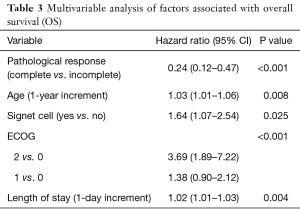
On multivariable analysis for OS and PFS as shown in Table 3, the timing of esophagectomy was not an independent variable associated with improvement in either OS or PFS. In contrast, factors that were independently associated with improved survival included lower age, absence of signet cell histology, better ECOG status, shorter post-operative length of stay and cPR. Thus, timing of esophagectomy was not associated with either survival outcomes or cPR as discussed above.
Full table
Discussion
There is evidence from prospective randomized trials to support the use of nCRT in esophageal cancer (9). It not only improves survival in select patients, but its benefits exceed the outcomes achieved by other approaches such as surgery alone or adjuvant chemoradiation (18,19). While this approach is particularly relevant in patients with T2 or higher lesions and those with regional lymph node disease, the question arises what is the optimal time to perform esophagectomy after the completion of nCRT. To address this, we reviewed our series and put forward hypothesis that since cPR has been shown to impact survival (17), then delaying esophagectomy after completion of nCRT could result in improved OS and PFS.
We had 226 patients available for our analysis, and 47 patients (20.8%) achieved cPR after nCRT. When comparing our patients based on the timing of esophagectomy (≤50 and >50 days), the timing of esophagectomy was not significantly associated with either cPR or OS and PFS. Thus, our hypothesis was not supported by this study’s results, and this is essentially a negative study. These findings were similar to some of the other studies that have also shown that timing of esophagectomy does not affect the rate of cPR (10,12). However, what is more unique to our study is that we used a Cox regression model to investigate the association between survival outcomes (OS and PFS), cPR and timing of esophagectomy as a continuous variable (Table 2). This showed that the timing of esophagectomy did not interact with pathological response. Delaying esophagectomy does not improve the chances of achieving a cPR. Thus, although this is a negative study, the findings are nonetheless important and clinically relevant because these data provide evidence that esophagectomy can be delayed following nCRT without detrimental effects to cPR or long-term survival outcomes.
cPR may be considered as a function of underlying tumor biology. Therefore, tumor biology is likely playing a much larger role in cPR and OS than the timing of esophagectomy after nCRT, whereby the interval from nCRT and surgery is much less consequential than the inherent aggressiveness of the cancer. With the development of modern, high throughput techniques, advances have been made in identifying aggressive genotypes for both esophageal adenocarcinoma and SCC. Several biomarkers have been proposed to have prognostic significance for patients with esophageal adenocarcinoma (20,21). Likewise, studies have identified potential prognostic biomarkers associated with aggressive subtypes of SCC (22-25). While these studies require validation in large, human cohorts, the fundamental principle remains that intrinsic characteristics of individual tumors contribute to differences in cPR observed within a cohort of patients, including our own. Differences in cPR to nCRT are likely reflective of underlying tumor biology, and as our study suggests, the timing of surgery following nCRT does not have a significant impact on this underlying tumor behavior.
We recognize that our study has limitations. First, this study is a retrospective analysis and is subject to selection bias that could have an influence on these results. Further, we did not elaborate on why some patients underwent esophagectomy quite late (>50 days). This is because the goal of our study was not focused at answering this question, but rather directed at determining whether the timing of esophagectomy had a detrimental impact on cPR or survival. There are a variety of reasons why patients underwent surgery at different time frames including scheduling logistics, medical clearance, and poor recovery from nCRT. However, regardless of the reason, delayed surgery was not associated with worse outcomes with respect to cPR or survival.
In summary, our results show that although cPR is predictive of improved survival (both OS and PFS), cPR is independent of the timing of esophagectomy. The timing of surgery therefore does not seem to overcome the underlying tumor biology in these patients. Although this is a negative study, the results are nonetheless important as they have relevant implications on clinical decision-making for patients with esophageal cancer undergoing multi-modal therapy. Specifically, our findings support the delay of esophagectomy following nCRT when sufficiently warranted (for example, by the need for prolonged post-nCRT recovery) without detriment to oncologic outcomes.
Acknowledgements
Funding: This work was supported by NCI grant P30CA016056 involving the use of RPCI’s Biostatistics Shared Resource.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The study was approved by Institutional Review Board of Roswell Park Cancer Institute (BDR035013). Informed consent was deemed unnecessary for this study as this was a retrospective review posing minimal risk to patients and consent could not be obtained for many patients in the study period.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Kirby TJ, Rice TW. The epidemiology of esophageal carcinoma. The changing face of a disease. Chest Surg Clin N Am 1994;4:217-25. [PubMed]
- National Comprehensive Cancer Network. Colon Cancer (Version 4.2014). Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg 1992;16:1104-9; discussion 1110. [Crossref] [PubMed]
- Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol 1993;11:1118-23. [Crossref] [PubMed]
- Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994;73:1779-84. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Tessier W, Gronnier C, Messager M, et al. Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? Ann Thorac Surg 2014;97:1181-9. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Kim JY, Correa AM, Vaporciyan AA, et al. Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg 2012;93:207-12; discussion 212-3. [Crossref] [PubMed]
- Tulchinsky H, Shmueli E, Figer A, et al. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15:2661-7. [Crossref] [PubMed]
- Petrelli F, Sgroi G, Sarti E, et al. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg 2016;263:458-64. [Crossref] [PubMed]
- Ruol A, Rizzetto C, Castoro C, et al. Interval between neoadjuvant chemoradiotherapy and surgery for squamous cell carcinoma of the thoracic esophagus: does delayed surgery have an impact on outcome? Ann Surg 2010;252:788-96. [Crossref] [PubMed]
- Shapiro J, van Hagen P, Lingsma HF, et al. Prolonged time to surgery after neoadjuvant chemoradiotherapy increases histopathological response without affecting survival in patients with esophageal or junctional cancer. Ann Surg 2014;260:807-13; discussion 813-4. [Crossref] [PubMed]
- Alnaji RM, Du W, Gabriel E, et al. Pathologic Complete Response Is an Independent Predictor of Improved Survival Following Neoadjuvant Chemoradiation for Esophageal Adenocarcinoma. J Gastrointest Surg 2016;20:1541-6. [Crossref] [PubMed]
- Gabriel E, Attwood K, Du W, et al. Association Between Clinically Staged Node-Negative Esophageal Adenocarcinoma and Overall Survival Benefit From Neoadjuvant Chemoradiation. JAMA Surg 2016;151:234-45. [Crossref] [PubMed]
- Gabriel E, Attwood K, Shah R, et al. Novel Calculator to Estimate Overall Survival Benefit from Neoadjuvant Chemoradiation in Patients with Esophageal Adenocarcinoma. J Am Coll Surg 2017;224:884-94.e1. [Crossref] [PubMed]
- Ong CA, Shapiro J, Nason KS, et al. Three-gene immunohistochemical panel adds to clinical staging algorithms to predict prognosis for patients with esophageal adenocarcinoma. J Clin Oncol 2013;31:1576-82. [Crossref] [PubMed]
- Adams O, Dislich B, Berezowska S, et al. Prognostic relevance of autophagy markers LC3B and p62 in esophageal adenocarcinomas. Oncotarget 2016;7:39241-55. [Crossref] [PubMed]
- Jiang YY, Lin DC, Mayakonda A, et al. Targeting super-enhancer-associated oncogenes in oesophageal squamous cell carcinoma. Gut 2017;66:1358-68. [Crossref] [PubMed]
- Sato-Kuwabara Y, Fregnani JH, Jampietro J, et al. Comparative analysis of basaloid and conventional squamous cell carcinomas of the esophagus: prognostic relevance of clinicopathological features and protein expression. Tumour Biol 2016;37:6691-9. [Crossref] [PubMed]
- Mallak AJ, Abbaszadegan MR, Khorasanizadeh PN, et al. Contribution of EVX1 in Aggressiveness of Esophageal Squamous Cell Carcinoma. Pathol Oncol Res 2016;22:341-7. [Crossref] [PubMed]
- Togashi Y, Arao T, Kato H, et al. Frequent amplification of ORAOV1 gene in esophageal squamous cell cancer promotes an aggressive phenotype via proline metabolism and ROS production. Oncotarget 2014;5:2962-73. [Crossref] [PubMed]


