Rectal and anal canal neuroendocrine tumours
Introduction
Neuroendocrine cells are present in a higher percentage in the gastrointestinal tract. Neuroendocrine tumors (NETs) are rare, representing about 0.5% of all newly diagnosed malignancies. Rectal (Rec) and anal canal (AC) NETs account for less than 1% of all Rec and AC cancers. The proportion of Rec NENs in Europe is less at 5–14% of all NETs (1-4). These tumors are often asymptomatic at diagnosis, being detected incidentally during routine lower gastrointestinal endoscopy. They present as small, sessile masses or thickened areas. The classic carcinoid symptoms occur in less than 10% of all patients and in most cases, requires hepatic metastatic disease (5). There are three histological groups: NETs, neuroendocrine carcinoid (NEC) tumors and mixed adenoneuroendocrine carcinoid tumors (MANEC). These are graded into three levels based on tumor cell proliferation: G1: Ki67 <3%; G2: Ki67 3–20%; G3: Ki67 >20%. NETs have well-differentiated with low cellular atypia—G1 or G2—and express neuroendocrine markers—chromogranin A, synaptophysin—and hormones. On the other hand, NEC is a poorly differentiated, high-grade malignant tumor with marked cellular atypia, frequent necrosis and high proliferative activity—G3 (6). The prognostic factors for metastatic disease are tumor size, depth on invasion and lymph node involvement (1,6,7).
Materials and methods
The study group was identified from the Portuguese Regional South Oncological Registry. From 2000 to 2014 twenty-two patients were identified with Rec and AC NETs treated at our institution. Medical records were retrospectively reviewed. Demographic, histological treatment features and oncologic outcomes were analyzed. The staging was based on the World Health Organization (WHO) and the 2012 European Neuroendocrine Tumour Society (ENETS) proposal (7). It was studied the use of endoscopic and surgical procedures, conventional laparoscopic surgery and the use of chemotherapy (CT) and radiotherapy (RT).
Statistical analysis
Due to the low incidence and fewness of these tumors, it was made a descriptive analysis and survivals outcomes, although without any statistical significance. It was utilized the IBM SPSS Statistics® software version 20.
Results
Twenty-two patients were identified and treated at our institution over a period of fifteen years [2000–2014], 54.5% were males and 45.5% females, with a median age at diagnosis of 59 years (range, 34–89 years). Most patients had Rec tumors (81.8%), although all AC were NEC tumors. The histological and staging features are categorized on Table 1.
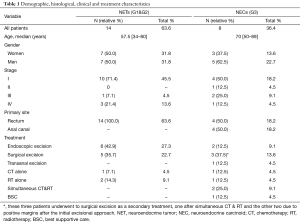
Full table
Patients with NEC tumors were treated with RT alone, simultaneous CT and RT followed by endoscopic excisional treatment, transanal excision, abdominoperineal (APR)/anterior rectum resection (ARR) when an R1 disease was present, ARR alone or Best Supportive Care. Patients with NETs were mostly treated with a resection approach either endoscopic or surgical.
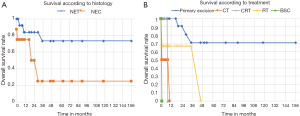
According to our results (Figure 1A), patients with NEC tend to have a poorer prognosis and lower survival, although we couldn’t identify significant differences between histology (P=0.67). Patients with primary excision as initial treatment (Figure 1B), had longer overall survival (P<0.05). The median time to progression was 12.4 months and the most frequent site of metastasis was the liver. For metastatic disease, the core treatment was CT although with poor response.
Discussion
Even though our cohort has a small number of cases it is important to emphasize Rec and AC NECs account for less than 0.1% of all colorectal malignancies and they are consistently poorly differentiated and associated with a poor prognosis (1-4). Our results show that NECs tend to have lower overall survival when compared with NETs and surgical or endoscopic tumor removal has a major impact on overall survival (Figure 1B), regardless the histological characteristics.
The risk of metastatic disease of NETs increases with tumour size—2% when <1 cm, 10–15% 1–2 cm, 60–80% ≥2 cm—with no involvement of the muscularis propria (8). This reiterates the importance of endoscopy ultrasonography for proper staging in tumors have <2 cm. NETs are often localized—75–85%—and rarely have distant metastasis at diagnosis—2–8%. The management of metastatic NETs, in this case, Rec NETs, experience shows that these are often very aggressive. There are two approved drugs to be applied in this setting, lanreotide, and everolimus (8,9). Some phase II studies also show some evidence of activity for VEGF inhibitors (10).
In locoregional NECs, emerging data suggest that chemoradiation without surgery may be sufficient, although still controversial. This is an important consideration given that a radical surgery, such as APR, maybe required. In the scope of metastatic disease, second line regimens after platinum based therapy are unclear.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Aytac E, Ozdemir Y, Ozuner G. Long term outcomes of neuroendocrine carcinomas (high-grade neuroendocrine tumors) of the colon, rectum, and anal canal. J Visc Surg 2014;151:3-7. [Crossref] [PubMed]
- Gaffey MJ, Mills SE, Lack EE. Neuroendocrine carcinoma of the colon and rectum. A clinicopathologic, ultrastructural, and immunohistochemical study of 24 cases. Am J Surg Pathol 1990;14:1010-23. [Crossref] [PubMed]
- Bernick PE, Klimstra DS, Shia J, et al. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum 2004;47:163-9. [Crossref] [PubMed]
- Chung TP, Hunt SR. Carcinoid and neuroendocrine tumors of the colon and rectum. Clin Colon Rectal Surg 2006;19:45-8. [Crossref] [PubMed]
- Shafqat H, Ali S, Salhab M, et al. Survival of patients with neuroendocrine carcinoma of the colon and rectum: a population-based analysis. Dis Colon Rectum 2015;58:294-303. [Crossref] [PubMed]
- Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013;42:557-77. [Crossref] [PubMed]
- Caplin M, Sundin A, Nillson O, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology 2012;95:88-97. [Crossref] [PubMed]
- Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387:968-77. [Crossref] [PubMed]
- Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-33. [Crossref] [PubMed]
- Chan JA, Kulke MH. Progress in the treatment of neuroendocrine tumors. Curr Oncol Rep 2009;11:193-9. [Crossref] [PubMed]


