Short course radiation as a component of definitive multidisciplinary treatment for select patients with metastatic rectal adenocarcinoma
Introduction
Though not commonly utilized in the United States, short course radiation therapy (SCRT) has been established as a standard option for the treatment of localized rectal adenocarcinoma in national and international guidelines (1,2). Several European trials showed the local control (LC) benefit of radiation and surgery compared with surgery alone (3-6). Pre-operative SCRT also showed an LC benefit also when compared with selective post-operative long-course CRT (7). More recently the TROG 01.04 randomized trial compared preoperative long-course CRT to preoperative SCRT followed by post-operative chemotherapy and found that, for patients with T3N0-2M0 rectal adenocarcinoma, there was no difference in 3-year LC, overall survival (OS), late toxicity rates or health-related quality of life (8,9).
For patients with metastatic disease at diagnosis, the treatment recommendations are less clear. This clinical scenario is not entirely uncommon, as approximately 15% of patients with rectal cancer present with liver metastases at diagnosis (10). Other sites of oligometastatic disease can include the lung, ovaries and distant lymph nodes (11). For patients with potentially resectable metastases, current National Comprehensive Cancer Center guidelines recommend upfront chemotherapy with or without preoperative long-course CRT followed by a staged or synchronous resection of both the primary and the metastases (12). However, 5 to 6 weeks of long-course CRT followed by a 6–8 weeks interval between completion of radiation and surgery may allow for disease progression due to lack of exposure to combination systemic therapy during this period. Thus, the American College of Radiology appropriateness guidelines state that preoperative SCRT can be considered for patients with metastatic disease for whom definitive management of the primary is desired (13), and a recent EORTC consensus statement on M1 rectal cancer actually recommends preoperative SCRT in this setting (14).
The optimal duration and sequence of therapy is unknown for potentially curable metastatic rectal adenocarcinoma. At our institution, all such cases are discussed in the multidisciplinary setting. We have adopted a strategy that includes neoadjuvant chemotherapy followed by SCRT and either synchronous or staged resection of the rectal primary as well as the metastatic disease. The purpose of this study is to assess outcomes and prognostic factors for progression-free survival (PFS) and OS following multidisciplinary treatment including SCRT among patients with metastatic rectal cancer.
Methods
Patient selection
The medical records of 34 patients with newly diagnosed M1 rectal adenocarcinoma who were treated with 25 Gray (Gy) in five fractions with definitive intent between 2010 and 2016 were identified. Patients with M1 disease were selected for a curative treatment course if they had a good performance status, pelvic disease amenable to complete resection and one or more metastatic sites amenable to surgical resection or other ablative/definitive treatment.
Multidisciplinary treatment course
Patients who presented with high-grade obstruction at diagnosis were surgically diverted upfront. Otherwise, most patients received upfront chemotherapy with a doublet or triplet 5-fluorouracil (5FU)-based regimen followed by a SCRT consisting of 5 Gy daily to 25 Gy. Surgical resection of the primary tumor followed when possible. Surgical management of the metastatic disease was performed either at the time of pelvic surgery or as a staged procedure.
Radiation details
All patients underwent a non-contrast CT simulation and were positioned prone on a belly board. The gross tumor volume (GTV) was defined as the primary tumor and any malignant-appearing lymphadenopathy. The planning target volume typically included the GTV with a 2 cm margin plus elective nodal coverage including the perirectal, presacral and internal iliac lymph nodes. A 3D conformal technique utilizing a posterior-anterior beam and two opposed laterals (3-field belly board; 3FBB) was favored at our institution until 2013. After 2013, the majority of patients were treated with intensity modulated radiation therapy (IMRT). Daily kilovoltage (kV) X-rays were used for image guidance.
Assessment of cancer control and toxicity endpoints
Patients were evaluated at least once during the course of radiotherapy for acute toxicities. Patients were also evaluated in their preoperative appointments, when applicable. Radiation-related toxicities were graded based on the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Acute toxicities were defined as those occurring between the initiation of radiation and the date of surgery or 6 weeks after the initiation of radiation (whichever was earlier). Post-operative complications were also recorded from the medical record and were classified as either potentially radiation-related (i.e., post-operative abscess) or not potentially radiation-related (i.e., pulmonary embolism or myocardial infarction). Dates and locations of progressive disease were recorded as well as vital status at last follow-up.
Statistical analysis
Chi-square test was used for between-group comparisons of categorical variables, and the Mann-Whitney U test was used for between-group comparisons of continuous variables. P values <0.05 were considered significant. PFS was calculated as the date of first treatment initiation to the date of disease progression, relapse, and death from any cause or last follow-up. OS was calculated as the date of first treatment initiation to the date of death from any cause or last follow-up. Analysis of PFS and OS was performed by the Kaplan-Meier method. Cox’s proportional hazards model was used for univariate and multivariate analyses to evaluate potential prognostic factors for PFS and OS. The hazard ratio (HR) is reported with the 95% confidence interval (CI) for each variable. Factors with a P value <0.2 on univariate analysis were included in the multivariate model. The statistical software used was JMP version 12 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 34 patients were included in the analysis. The median [interquartile range (IQR)] follow up was 25 (14.75–42.25) months. All but one patient received upfront chemotherapy prior to SCRT; 26 patients went on to receive definitive surgery for their rectal primary disease. Eight patients had their surgeries cancelled; four were cancelled due to progression of metastatic disease, two were cancelled due to worsening of their medical comorbidities and two refused surgical intervention after an excellent clinical response and negative biopsy. Twenty-two patients received surgical resection of metastatic disease either at the time of resection of their rectal primary or in staged procedures, and at two patients received radiofrequency ablation (RFA) of metastatic disease with curative intent. Seven patients did not have curative treatment for their metastatic disease due to progression of metastases seen on re-staging imaging. Further patient, disease and treatment-related details are given in Table 1.
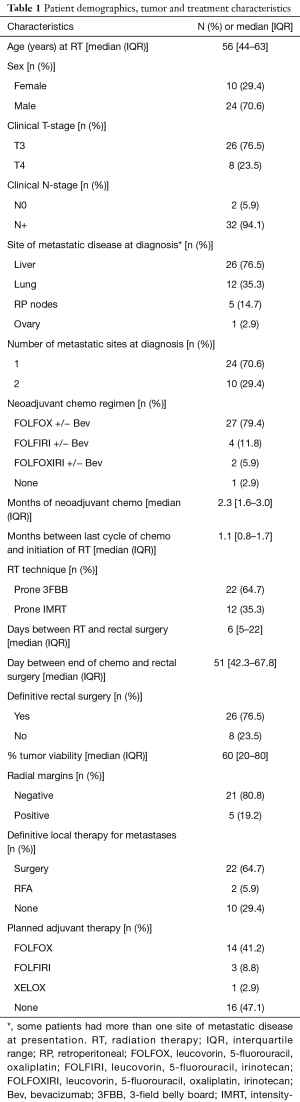
Full table
OS and PFS
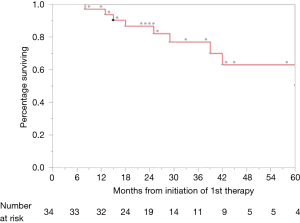
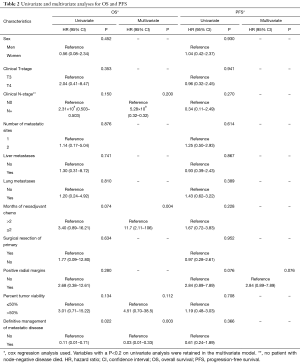
At the time of last follow up in June 2017, nine patients (26.5%) had died. One-, 2- and 3-year OS rates were 97%, 86.2% and 76.0%, respectively (Figure 1). On log-rank testing, there was no difference in OS between those who received definitive rectal surgery and those who did not (P=0.533); however, only 8 (23.5%) did not receive definitive rectal surgery, and only one of those eight had died at the time of analysis. On multivariate analysis, definitive management of metastases was associated with improved OS (HR 0.03, 95% CI: 0.01–0.33; P=0.003), and ≤2 months of neoadjuvant chemotherapy was associated with decreased OS (HR 11.7, 95% CI: 2.11–106; P=0.004) (Table 2).
Full table
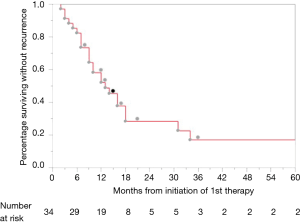
At the time of last follow up in June 2017, 25 patients (73.5%) had experienced progression of disease. One-, 2-, and 3-year PFS were 52.1%, 22.7% and 17%, respectively (Figure 2). Twenty-four patients experienced distant disease progression only and one patient experienced local and distant disease progression. For those who progressed, median interquartile range (IQR) time to progression was 12 (7–17.25) months. There were no factors identified on multivariable analysis that were significantly associated with differences in PFS; however, positive radial margins, typically an indicator of advanced primary disease, was associated with a trend towards decreased PFS (HR 2.84, 95% CI: 0.89–7.89; P=0.076) (Table 2).
Acute toxicity and post-operative complications
Six patients (17.6%) developed grade 2 or greater toxicities from the initiation of radiation until the date of surgery or 6 weeks after radiation (whichever came first). One patient developed grade 3 diarrhea, two patients developed grade 2 diarrhea, and three patients developed grade 2 nausea. Of the 26 patients who underwent definitive surgery for their primary tumor, 10 (40%) developed potentially radiation-related postoperative complications. Postoperative complications included three patients with small bowel obstruction, five patients with pelvic abscess or wound infection, one patient with high ostomy output and dehydration and one patient with an anastomotic leak requiring surgical correction.
Radiation technique, dosimetric endpoints and toxicity
Patients who received treatment with IMRT had a significantly lower volume of bowel receiving 25 Gy or greater (V25), as well as a lower V20 and V15 (Table 3). However, there was no demonstrated significant correlation with IMRT use and lower toxicity compared with 3FBB.
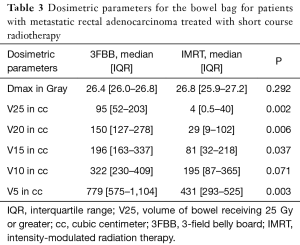
Full table
Discussion
Our results suggest that outcomes are encouraging for select patients with metastatic rectal cancer treated with a definitive multidisciplinary approach that included chemotherapy followed by SCRT followed by pelvic surgery a median IQR of 6 [5–22] days later. Given the observed benefit of “short course, long interval” treatment outlined above for patients with localized rectal cancer (15-17), there is likewise interest in incorporating a planned break between SCRT and pelvic surgery for patients with metastatic disease during which additional chemotherapy can be administered.
Although two patients in our cohort experienced a clinical complete response and refused surgery, no patient in our cohort experienced a pCR. Similarly low pCR rates have been reported in other SCRT series including immediate surgery. The TROG 01.04 trial showed similarly low pCR rates of only 1% for patients receiving SCRT followed by immediate surgery (8,18). The timing of surgery with respect to SCRT has been the topic of ongoing investigation, and protocols using SCRT followed by delayed surgery (4–8 weeks interval) have shown to improve tumor response compared to the traditional 1–2 weeks interval (15,16). Interestingly, preliminary data from a recent Polish trial showed comparable LC and potentially improved OS with SCRT followed by FOLFOX×4 prior to surgery compared to long-course CRT with bolus 5FU/leucovorin and oxaliplatin (17).
This approach of SCRT followed by chemotherapy for up to six cycles followed by surgery was the subject of a phase II study in the Netherlands. The 2-year recurrence rate was relatively high at 64% (19,20). Our results compare favorably to these, as our R0 resection rate was 80.2%, our 2-year OS was 86.2% and our 2-year progression rate was 77.3%. A similar approach was taken by a Phase II study of liver-metastasis only rectal cancer patients in Korea. The R0 rate was 63%, which, again, is lower than in our series (80.8%) (21).
Interestingly, receipt of definitive pelvic surgery was not significantly associated with either OS or PFS in our cohort. This is likely due to the high rate of distant failure being the primary driver of outcomes. Additionally, eight patients had their pelvic surgery cancelled (n=8), two of which had a clinical complete response to preoperative therapy. None of the eight patients receiving SCRT alone without subsequent pelvic surgery developed late toxicities related to radiation, and these results are in keeping with a phase 2 study evaluating SCRT for palliative intent (22). We did not see any differences in toxicity between patients treated with 3DCRT compared with those treated with IMRT; however, IMRT was able to decrease the volume of bowel receiving low doses of radiation. Other groups have also shown that IMRT can be beneficial in decreasing small bowel dose as well as bone marrow dose and resultant toxicities (23,24).
All patients in our study received a fluorouracil-based doublet or triplet regimen with or without bevacizumab. Patients received two to six cycles of chemotherapy prior to SCRT, and our results suggest that receipt of >2 months of neoadjuvant chemotherapy was associated with improved OS on multivariate analysis. This may be due to more adequate treatment of systemic disease before adding local therapy, or it may be that a longer course of neoadjuvant chemotherapy allows for improved patient selection. This is an area that may warrant further research.
This single-institution, retrospective study is the first US report of outcomes and prognostic factors for patients treated with curative intent using SCRT as part of the multidisciplinary management of metastatic rectal cancer. These results are hypothesis-generating and suggest that definitive management of metastatic sites with surgery or other local therapy as well as receipt of >2 months of induction chemo prior to local therapy are important prognostic factors for survival. Limitations of this study include those inherent to any small retrospective review. There were certainly patient and disease related factors that influenced the multidisciplinary team to offer definitive treatment. Additionally, there was some heterogeneity in the timing and agents used for the systemic therapy component.
Conclusions
In conclusion, patients with potentially curable metastatic rectal cancer can be effectively treated with a multimodality approach incorporating chemotherapy, SCRT and surgical resection. Though disease progression is common, particularly distantly, long-term control and survival are possible. Further study is warranted regarding the optimal sequence of chemotherapy, radiation and surgery of both the primary and metastatic sites.
Acknowledgements
None.
Footnote
Conflicts of Interest: These data will be presented in abstract form at the 2017 Meeting for the American Society for Radiation Oncology (ASTRO) in San Diego, California, USA.
Ethical Statement: The study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center (NO. #PA16-0654) and a waiver of informed consent was granted by the board in order to carry out this work.
References
- NCCN. National Comprehensive Cancer Center Network Guidelines Version 3.2017.
- Glimelius B, Tiret E, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi81-8. [Crossref] [PubMed]
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. [Crossref] [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644-50. [Crossref] [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [Crossref] [PubMed]
- Cedermark B, Johansson H, Rutqvist LE, et al. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer 1995;75:2269-75. [Crossref] [PubMed]
- Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811-20. [Crossref] [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2013;30:399. [PubMed]
- McLachlan SA, Fisher RJ, Zalcberg J, et al. The impact on health-related quality of life in the first 12 months: A randomised comparison of preoperative short-course radiation versus long-course chemoradiation for T3 rectal cancer (Trans-Tasman Radiation Oncology Group Trial 01.04). Eur J Cancer 2016;55:15-26. [Crossref] [PubMed]
- Mantke R, Schmidt U, Wolff S, et al. Incidence of synchronous liver metastases in patients with colorectal cancer in relationship to clinico-pathologic characteristics. Results of a German prospective multicentre observational study. Eur J Surg Oncol 2012;38:259-65. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6:29765. [Crossref] [PubMed]
- Anon. National Comprehensive Cancer Center Network Guidelines Version 2.2016 Rectal Cancer.
- Goodman KA, Milgrom SA, Herman JM, et al. ACR Appropriateness Criteria® rectal cancer: metastatic disease at presentation. Oncology (Williston Park) 2014;28:867-71, 876, 878. [PubMed]
- Lutz MP, Zalcberg JR, Glynne-Jones R, et al. Second St. Second St. Gallen European Organisation for Research and Treatment of Cancer Gastrointestinal Cancer Conference: consensus recommendations on controversial issues in the primary treatment of rectal cancer. Eur J Cancer 2016;63:11-24. [Crossref] [PubMed]
- Pettersson D, Lörinc E, Holm T, et al. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg 2015;102:972-8; discussion 978. [Crossref] [PubMed]
- Latkauskas T, Pauzas H, Gineikiene I, et al. Initial results of a randomized controlled trial comparing clinical and pathological downstaging of rectal cancer after preoperative short-course radiotherapy or long-term chemoradiotherapy, both with delayed surgery. Colorectal Dis 2012;14:294-8. [Crossref] [PubMed]
- Bujko K. Neoadjuvant Chemoradiation for Fixed cT3 or cT4 Rectal Cancer: Results of a Polish II Multicentre Phase III Study. J Clin Oncol 2016;34:abstr 489.
- Roy A, Mahasittiwat P, Weiner AA, et al. Preoperative short-course radiation therapy for rectal cancer provides excellent disease control and toxicity: Results from a single US institution. Pract Radiat Oncol 2017;7:e51-e58. [Crossref] [PubMed]
- van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol 2013;24:1762-9. [Crossref] [PubMed]
- Bisschop C, van Dijk TH, Beukema JC, et al. Short-Course Radiotherapy Followed by Neoadjuvant Bevacizumab, Capecitabine, and Oxaliplatin and Subsequent Radical Treatment in Primary Stage IV Rectal Cancer: Long-Term Results of a Phase II Study. Ann Surg Oncol 2017;24:2632-8. [Crossref] [PubMed]
- Kim KH, Shin SJ, Cho MS, et al. A phase II study of preoperative mFOLFOX6 with short-course radiotherapy in patients with locally advanced rectal cancer and liver-only metastasis. Radiother Oncol 2016;118:369-74. [Crossref] [PubMed]
- Picardi V, Deodato F, Guido A, et al. Palliative Short-Course Radiation Therapy in Rectal Cancer: A Phase 2 Study. Int J Radiat Oncol Biol Phys 2016;95:1184-90. [Crossref] [PubMed]
- Mok H, Crane CH, Palmer MB, et al. Intensity modulated radiation therapy (IMRT): differences in target volumes and improvement in clinically relevant doses to small bowel in rectal carcinoma. Radiat Oncol 2011;8:6:63.
- Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys 2013;86:83-90. [Crossref] [PubMed]


