Pacritinib to inhibit JAK/STAT signaling in refractory metastatic colon and rectal cancer
Introduction
Treatment options for patients with metastatic colon and rectal cancer (CRC) that have progressed following standard first and second lines of treatment are limited. The approvals of regorafenib and TAS-102 filled a void; however, results are disappointing for most patients (1,2). Patients with microsatellite unstable tumors may benefit from immunotherapy, however this represents a minority of cases (3). Novel approaches are needed for patients with refractory CRC (4).
Pre-clinical studies have demonstrated that cytokine mediated inflammation plays a role in carcinogenesis and prognosis in CRC (5). Interleukin-6 (IL-6) is an inflammatory cytokine that binds to receptors that mediate signaling by the JAK/STAT pathway. Phosphorylated STAT3 is a transcription promoter of genes associated with cell survival, proliferation, angiogenesis, metastasis, cell adhesion, and inflammation (6). Inhibiting this pathway in both CRC cell lines and mouse CRC xenograft models resulted in apoptosis and tumor responses (7).
Pacritinib is an orally administered multi-kinase inhibitor with potent JAK and FLT3 inhibition. It has been extensively evaluated in early phase trials in hematologic malignancies including myelofibrosis, AML, and myeloproliferative neoplasms and shown to have a favorable safety profile (8).
This phase II single arm, single institutional trial sought to examine the effect of JAK/STAT inhibition with pacritinib in patients with refractory colorectal cancers.
Methods
Eligibility criteria
Eligible patients had metastatic CRC refractory or intolerant to at least two lines of chemotherapy and evaluable or measurable disease by RECIST version 1.1, and Eastern Cooperative Oncology Group performance status of 0 or 1. Patients also had to be ≥18 years old with adequate bone marrow, hepatic and renal function as well as life expectancy of at least 3 months. Key exclusion criteria included prior therapy with JAK or FLT3 inhibitors, chemotherapy or targeted agent within 2 weeks of initiating study drug, known brain metastasis, or uncontrolled intercurrent illness. The protocol (ClinicalTrials.gov identifier: NCT02277093) was approved by the institutional review board of Washington University (IRB00009237) and written informed consent was obtained for all patients before performing study-related procedures.
Drug administration and study design
Pacritinib was administered orally continuously on a 28-day cycle at a dose of 200 mg twice daily. This dose was determined based on published phase one studies of pacritinib in patients with myeloid malignancies and myeloproliferative neoplasms (9). Patients underwent a baseline history and physical examination, ECG including QTc measurement, CT chest/abdomen/pelvis within 4 weeks, and CBC, CMP, and magnesium measurement. While on treatment patients were monitored with weekly blood work and every other week office visits and ECG with QTc measurement. Response assessment was by CT scans every two cycles or sooner if deemed necessary by the treating physician.
Correlative studies
Planned correlative studies included measurement of erythrocyte sedimentation rate and C-reactive protein. Serum IL-6 and interleukin-8 (IL-8) levels were also measured at baseline and monthly on treatment.
Statistical considerations
We proposed a meaningful primary outcome favoring continued development of pacritinib in refractory CRC was a median PFS of 4 months. The planned sample size of 38 patients would have given the study ≥0.90 power to identify an increase in median PFS from 1.7 to 4.0 months.
Results
Patient population
Eleven patients with refractory metastatic colon or rectal cancer were enrolled between September 2015 and February 2016. Patient characteristics are presented in Table 1. Median age was 63 years. All patients had been treated with FOLFOX and FOLFIRI. The three RAS wildtype patients had received panitumumab and nine patients had received bevacizumab. Five patients had participated in other clinical trials. The baseline median CRP level was 12.1 mg/L with a range of 2.1 to 147 mg/L.
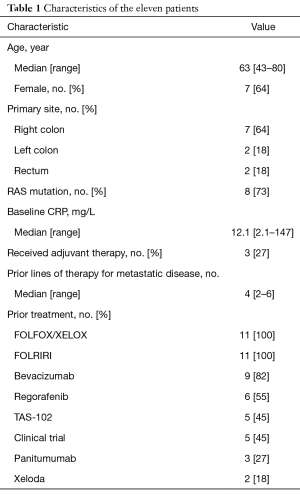
Full table
Adverse events
All patients experienced at least grade 1 or 2 adverse events. The most common being nausea (5 patients, 50%) followed by abdominal cramping and constipation. Five patients experienced a total of 14 unique grade 3 adverse events. There were no grade 4 or 5 events on study (Table 2).
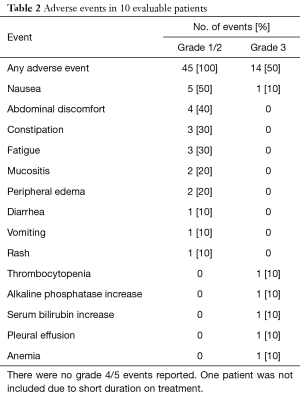
Full table
Correlative studies
CRP and interleukin concentrations at baseline and after the first cycle were available for 7 and 9 patients respectively. Interleukin 6 and 8 plasma levels were measured by Luminex high sensitivity multiplex assay. The patient with stable disease by RECIST at 2 months had the lowest baseline CRP of 2.1 mg/L. The patient with the highest CRP on study, 147 mg/L had a large burden of liver metastasis and an elevated total bilirubin not related to pacritinib. IL-6 and IL-8 levels increased in all patients after 1 month of treatment (Figure 1).
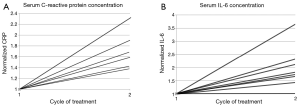
Antitumor efficacy
Seven patients were evaluable for response while four patients discontinued due to an FDA hold on pacritinib prior to first radiographic imaging. Of the 7 evaluable patients, 6 had disease progression while 1 had stable disease by RECIST criteria. No patient continued pacritinib for longer than 8 weeks.
Discussion
This study sought to demonstrate that inhibition of the JAK/STAT cytokine signaling pathway would lead to clinically meaningful responses in patients with refractory CRC. After eleven patients accrued and were initiated on treatment the Food and Drug Administration of the United States issued an order holding administration of pacritinib and halting clinical trial enrollment. This was based on emerging adverse safety data from the PERSIST-1 trial in patients with myelofibrosis treated with pacritinib. During the clinical hold we determined that re-opening the study with additional safety parameters was not in the best interest of our patients given the lack of observed response to the investigational agent and uncertainty as to the safety of pacritinib.
While we cannot evaluate the effect of pacritinib on our primary and secondary endpoints, several observations were made. The drug was only discontinued in one patient due to intolerance. There were no grade 4/5 events and no cardiovascular events were observed. The safety evaluation is limited by the short duration of treatment. Correlative analysis of inflammatory markers demonstrated an increase over the course of one month on treatment. Our opinion is that this finding is most likely related to progressive disease but the possibility of an interaction with pacritinib should be explored further. We were unable to find published reports of CRP, IL-6 or IL-8 measurement in patients treated with pacritinib or other JAK inhibitors.
The hypothesis that patients with high CRP levels would be more likely to respond to pacritinib was not supported by our observations. However, since no patient had an objective response to treatment this hypothesis cannot be evaluated.
The phase III CORRECT trial of regorafenib versus placebo found a small survival benefit in favor of regorafenib. Despite the modest difference, it supported a role for small-molecule multikinase inhibitors in the treatment of refractory colorectal cancer. Our median PFS expectation for pacritinib was deliberately set based on these results and pacritinib did not meet this endpoint. Further study of pacritinib as a single agent is not warranted based on our study, however; novel combinations with drugs targeting other molecular pathways could be explored.
Acknowledgements
This work was supported by CTI Biopharma for funding and providing pacritinib and The Alvin J Siteman Cancer Center and Washington University School of Medicine for additional support. The authors also acknowledge Stephanie Myles, Maureen Highkin, and Allison Creekmore for their support of this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol (ClinicalTrials.gov identifier: NCT02277093) was approved by the institutional review board of Washington University (IRB00009237) and written informed consent was obtained for all patients before performing study-related procedures.
References
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909-19. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol 2016;8:57-84. [Crossref] [PubMed]
- Knüpfer H, Preiss R.. Serum interleukin-6 levels in colorectal cancer patients--a summary of published results. Int J Colorectal Dis 2010;25:135-40. [Crossref] [PubMed]
- Yu H, Pardoll D, Jove R.. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009;9:798-809. [Crossref] [PubMed]
- Lin L, Liu AG, Peng ZG, et al. STAT3 Is Necessary for Proliferation and Survival in Colon Cancer-Initiating Cells. Cancer Research 2011;71:7226-37. [Crossref] [PubMed]
- Hatzimichael E, Tsolas E, Briasoulis E. Profile of pacritinib and its potential in the treatment of hematologic disorders. J Blood Med 2014;5:143-52. [Crossref] [PubMed]
- Verstovsek S, Komrokji RS. A comprehensive review of pacritinib in myelofibrosis. Future Oncol 2015;11:2819-30. [Crossref] [PubMed]


