Establishment and validation of prognostic nomograms in first-line metastatic gastric cancer patients
Introduction
Gastric cancer is the third leading cause of cancer-related death worldwide in 2012 (723,000, 8.8% of the total), and the highest estimated mortality rates are in Eastern Asia (1). Compared with the best supportive care, first-line chemotherapy for inoperable locally advanced or metastatic gastric cancer (AGC) improves survival and quality of life (2-4), but the median overall survival (OS) is short (9–13 months) (5-7).
Royal Marsden Hospital (RMH) prognostic index (RMH index), including Eastern Cooperative Oncology Group (ECOG) performance status (PS), liver metastasis, peritoneal metastasis and serum alkaline phosphatase (ALP) in the model, and the Japanese Clinical Oncology Group (JCOG) prognostic index (JCOG index), which includes the ECOG PS, number of metastatic sites, no prior gastrectomy, and serum ALP, have been reported (6,8). However, because these indexes were based on clinical trials, it has not been well documented whether we can apply such indexes to our clinical practice patients. Among AGC patients, the rate of human epidermal growth factor receptor 2 (HER2) positivity is approximately 10–30% (9,10). On the basis of the trastuzumab for gastric cancer (ToGA) trial results, addition of trastuzumab to platinum-based chemotherapy has become standard, first-line chemotherapy for HER2-positive AGC (11). However, it remains unclear whether HER2 status is an outcome-associated biomarker independent of known prognostic factors for AGC (12-14).
On the other hand, nomograms are widely used as tools to estimate survival probability tailored to individual patients, informing clinicians and patients to decide on treatment plans (15,16). Although some studies have reported nomograms for predicting the outcomes of gastric cancer after R0 resection or the survival of other cancer types, few reports are available on predicting the survival outcomes of AGC (17,18).
The aim of this retrospective study was to combine the HER2 status and other previously reported predictors for AGC in nomograms that would enable clinicians to estimate the survival outcomes of individual patients starting first-line chemotherapy.
Methods
Each Institutional Review Board at Aichi Cancer Center Hospital (ACC) and Shizuoka Cancer Center approved this study and granted the opt-out of informed consent, considering the retrospective nature of this analysis. The principal eligibility criteria for this study included patients with the presence of histologically confirmed inoperable gastric cancer. The patients of each institution underwent chemotherapy. We evaluated a training data set from ACC to establish nomograms to calculate the predicted probability of OS and progression free survival (PFS). The nomograms were validated using an independent dataset from SCC. Written informed consent for treatment was obtained from each patient prior to treatment initiation.
Training and validation set
The population of the training set comprised of consecutive patients with AGC who were treated at ACC between January 2005 and December 2012. The patients, all undergoing chemotherapy as first-line treatment (with or without targeted therapy), were included in this study. Specific details of the training population dataset have been reported in previous articles (13,19,20). The validation set consisted of consecutive patients with AGC who were treated at Shizuoka Cancer Center from January 2010 to December 2012.
HER2 evaluation
HER2 positivity was tested at each institution and defined as an IHC score of 3+ or an IHC score of 2+ and in situ hybridization (ISH)-positive by fluorescence ISH (FISH) or dual-color ISH (DISH), which are considered to be indications for using trastuzumab as per previous ToGA trial results (11,21).
Statistical analysis
The statistical significance of differences in proportions and medians were compared using independent χ2 tests for categorical variables. OS was calculated from the date of first-line therapy initiation to the date of death or last follow-up visit. Disease progression associated with first-line chemotherapy was also measured from the beginning of treatment to the date of disease progression, as evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Survival status and disease status were updated in May 2015.
To determine which prognostic factors should be included in the nomograms, we used Cox proportional hazards regression analyses by hazard ratios (HR) and 95% confidence intervals (CI) of the baseline characteristics to predict PFS probabilities at 3 and 6 months and 1 year and OS at 6 months and 1 and 2 years. A survey of the potential nomograms was carried out within the model, including five factors. The number of factors was determined by considering the applicability of the results to clinical practice and to avoid an over-fit model. We decided definitely to include the HER2 status, because recent HER2 positive AGC patients have a better prognosis than HER2 negative patients, especially when treated with trastuzumab (13). In addition, if there were correlation (rho value: >0.70 or <−0.70) among each baseline patient characteristics examined in the Spearman correlation test, we did not include these variables in our models. To construct a nomogram, we used the model coefficients, as estimated in the training set, for the discrimination and calibration analyses in the validation set via the Harrell’s C-index (22). The accuracy of the nomogram was assessed using both cohorts via calibration plots. For the calibration plots, we assessed by comparing the predicted probability versus the observed probability. Missing covariate data were estimated using multiple-imputation methods, including the start year of first-line chemotherapy as covariates to avoid bias due to the changes of standard chemotherapy regimens in Japan during this study. Cox regression analyses were fitted to imputed datasets and combined into a single model with averaged regression coefficients and variance and covariance estimates adjusted for imputations. To compare nomograms with the RMH index and JCOG index, the Cox proportional hazard models were performed in the training set, excluding the patients with missing data among the prognostic factors in the RMH index and JCOG index.
The statistical analyses were performed using the R software version 2.13.2 (R Project for Statistical Computing, Vienna, Austria). All of the tests were two-sided, and P values <0.05 were considered statistically significant.
Results
Patient characteristics
During the study period, we identified 838 consecutive patients in the training set and 269 patients in the validation set. Patient characteristics are summarized in Table 1. Patients in the training set had worse PS (0/1/≥2, 34%/51%/15% vs. 45%/44%/11%, P<0.01), poorer differentiated carcinoma (diffuse type, 69% vs. 58%, P<0.01), and a larger number of metastatic sites (1/≥2, 56%/44% vs. 44%/56%, P<0.01) than patients in validation set. First-line chemotherapy agents in the training set and the validation set were as follows: fluoropyrimidines (5-FU, S-1, and capecitabine), 87% vs. 90%; platinums (cisplatin and oxaliplatin), 54% vs. 71%; taxanes (paclitaxel and docetaxel), 14% vs. 5%; irinotecan, 5% vs. 6%; and trastuzumab, 6% vs. 4%. The rho values of the Spearman correlation test among baseline patient characteristics in the training set were from −0.70 to 0.70, all the variables did not correlate with each other. Details are shown in Table S1.
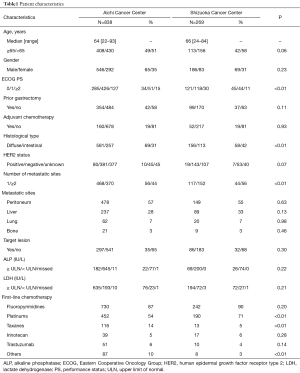
Full table

Full table
Survival outcomes
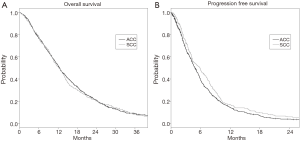
After median follow-up periods of 12.3 (range, 0.2–92.5) and 11.6 (range, 0.4–65.3) months, 782 patients (93.3%) and 248 patients (92.2%) died in the training set and validation set, respectively. Median OS was 12.5 months (95% CI, 11.8–13.2) in the training cohort and 12.4 months (95% CI, 10.6–13.8) in the validation cohort (P=0.67, Figure 1). Table 2 shows the results of the univariate analyses for OS in the training cohort, revealing that poor PS, no prior gastrectomy, diffuse type, HER2 negative, number of metastatic sites, metastasis of peritoneum, metastasis of lung, metastasis of bone, elevated ALP, elevated LDH each possessed a P value <0.05.
Full table
In addition, 757 and 247 patients experienced disease progression, respectively, in the training and validation datasets; PFS was longer in the validation cohort (4.8 vs. 5.8 months; P=0.03; Figure 1B), in line with the differences in chemotherapy regimens and the era of initiation of the first-line chemotherapy between the two cohorts. Table 2 shows the univariate analyses for PFS in the training cohort. Poor PS, no prior gastrectomy, HER2 negative status, number of metastatic sites, metastasis of liver, elevated ALP, and elevated LDH (P<0.01 for each factor) yielded poor prognosis. The details of the univariate analyses for OS and PFS in the validation cohort are shown in Table S2.

Full table
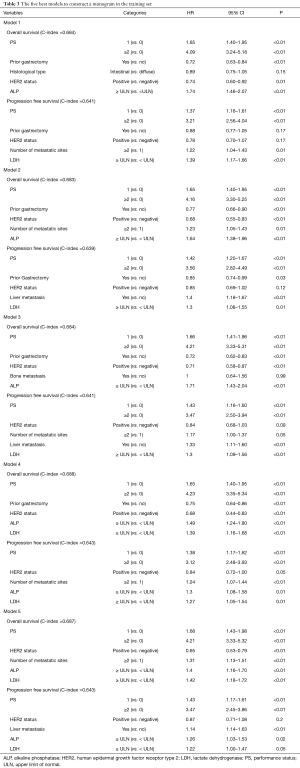
Prognostic nomograms
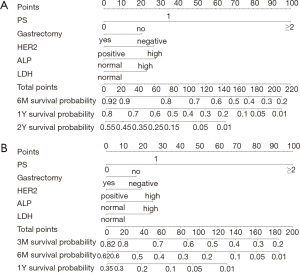
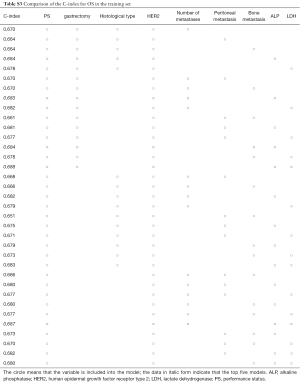
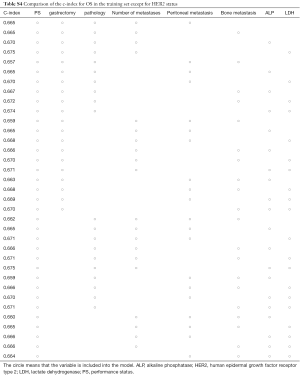
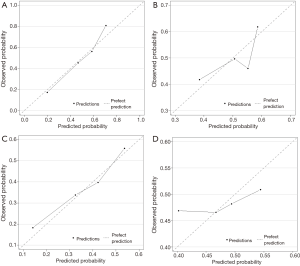
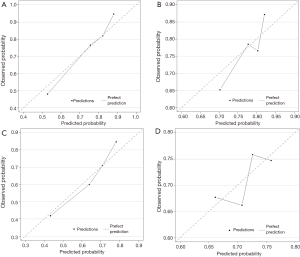
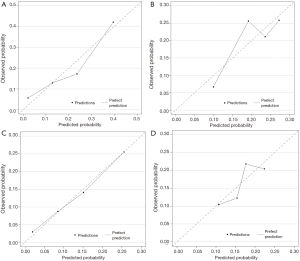
Figure 2 displays nomograms derived from the prognostic model and estimated probability of PFS at 3 and 6 months and 1 year and OS at 6 months and 1 and 2 years. The nomograms can be predicted at different time points for an individual patient. To construct the nomograms, we put forward the five best models of OS and PFS that the C-index values were highest in all possible models (Tables S3-S6). Comparing C-index values between these models was not informative, and we wanted to ensure the objectivity of diagnosis in a clinical setting and avoid differing diagnostic abilities among institutions when used in other hospitals. Thus, we selected model 4 for OS, which consisted of ECOG PS, prior gastrectomy, HER2 status, serum ALP, and serum LDH, and PFS, including ECOG PS, HER2 status, number of metastatic sites, serum ALP, and serum LDH (Table 3). The discriminatory ability of the established models was assessed in the training and validation samples. The Harrell’s C-indexes for OS were 0.688 (95% CI, 0.664–0.711) in the training set and 0.576 (0.534–0.618) in the validation set; for PFS, these values were 0.643 (95% CI, 0.617–0.688) and 0.544 (0.501–0.586). To evaluate nomogram accuracy, we plotted observed OS and PFS probabilities against the calculated estimated probabilities for each patient in the training and validation samples at different time points (Figure 3). The calibration plots showed well OS calibrations at 1 year and PFS at 6 months. Calibration plots at the other time points are shown in Figures S1 and S2.
Full table
Full table

Full table

Full table
Full table
In the training set, the C-index of the nomograms for OS was 0.607 (95% CI, 0.584–0.629) in the RMH index and 0.650 (95% CI, 0.626–0.673) in the JCOG index; for PFS, the values were 0.601 (95% CI, 0.577–0.624) and 0.611 (95% CI, 0.586–0.635), respectively for RMH and JCOG indices. Overall, in the validation cohort, these performances favored our models (Table S7).
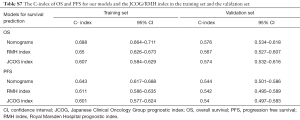
Full table
Discussion
To the best of our knowledge, nomograms are well established and externally validated to predict clinical outcomes in relatively large population of patients with AGC undergoing first-line chemotherapy at two different institutions. Moreover, our models are the first to combine the HER2 status as a prognostic variable with other well-established prognostic factors; we observed good performance of our models compared with the JCOG index (8) and the RMH index (6), in which a C-index value in excess of 0.015 is deemed clinically relevant (23). We believe that nomograms are crucial determinants of clinical care for individual AGC patients.
The nomograms for gastric cancer patients who were curatively resected have been reported (17,24); however, there are few reports about nomograms for AGC. Capanu et al. reported a clinical nomogram for AGC patients treated with systemic chemotherapy estimating the probability of surviving for 2 years, in which the variables were ECOG PS, liver metastases, baseline serum albumin, hemoglobin, age, histology, more than one metastatic site, and lymph node metastases (25). These variables, except for lymph node metastases, were already reported as independent prognostic factors and included in previous prognostic models for AGC. However, this study was focused on a statistical software model and was not externally validated. Another nomogram has been reported, including PS, histological grade, number of metastatic sites, bone metastases, ascites, neutrophil-to-lymphocyte ratio (NLR), and HER2-positive as predictors in only Caucasian patients (26). However, ascites and NLR are not common of prognostic factors for gastric cancer, and our nomogram is derived from Asian patients not from Caucasian patients.
Our models had good discrimination ability for OS, with a Harrell’s C-index of 0.688 in the training set and, in contrast, of 0.576 in the validation set. The calibration plots for OS at 6 months and 1 and 2 years were also good in both cohorts and could be considered validated. However, the models for PFS were less predictive compared to OS in both cohorts; however, good calibrated plots were examined in both cohorts at 3 and 6 months and 1 year. The differences between OS and PFS discrimination performance may be due to the fact that OS generally depends on tumor biology and tumor burden and is considered a true endpoint, whereas PFS relies on numerous variables of treatment response.
The C-indexes of the established nomograms were better than those of the RMH index and JCOG index in both training and validation cohorts. These previous models were established from clinical trial data, differing from our models. Our model may be useful and applicable for clinical care of individual patients.
Our models consisted of HER2 status to reflect the clinical management of HER2 positive gastric cancer via trastuzumab era; there were higher C-indexes upon the inclusion of HER2 status than not in the training set’s OS and PFS data. Treatment strategies are different between HER2 positive and negative status, and actually the design of recent prospective phase III trials in first-line therapy for AGC have been conducted with discrimination of HER2 positive status from HER2 negative status. However, it is controversial whether HER2 status is an independent prognostic factor for AGC patients undergoing first-line therapy. Janjigian et al., reported that HER2 positivity was not an independent prognostic factor compared with negativity (HR 0.79; 95% CI, 0.44–1.14; P=0.194). In contrast, it has been reported that HER2-positive patients treated with trastuzumab survived significantly longer than that of the HER2-negative patients, with an adjusted HR of 0.58 (13). This model also included serum LDH as a variable, and it is incongruent with the previous models of RMH index and JCOG index. In the report of RMH index, serum LDH was unmentioned; however, regarding the JCOG index, serum LDH was identified as an independent prognostic factor for AGC (HR 1.48), gleaned from a multivariate analysis, compared to ALP (HR 1.36). Actually, the χ2 value from 1 of the 5 best models to construct the JCOG index was higher than that of the established model (χ2 values: 86.992 vs. 86.311). Serum LDH has been reported as a prognostic factor for other cancer types such as lung cancer (27), colorectal cancer (28), and prostate cancer (29). Therefore, future nomogram development in AGC could evaluate HER2 status and serum LDH levels, which may also help predict OS for AGC.
A key limitation of this study stems from its retrospective nature; in addition, the HER2 status was missing from 45% and 40% of patients in the training and validation cohorts, respectively. Prognosis of HER2 positive patients may be affected upon trastuzumab use: standard chemotherapy regimens including trastuzumab use changed during the data collection period. However, missing variables were complemented using multiple-imputation methods, including the start year of first-line chemotherapy as covariates to avoid bias due to changes in standard chemotherapy regimens.
In conclusion, we developed and tested nomograms for estimating the OS and PFS of AGC patients receiving first-line chemotherapy. These nomograms can serve as a guide to inform clinical decisions concerning chemotherapy intensity/regimens for AGC patients.
Acknowledgements
The Authors would like to thank Enago (www.enago.jp) for the English language review.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Aichi Cancer Center Hospital (No. 2015-1-006) and Shizuoka Cancer Center (No. T27-28). Written informed consent for treatment was obtained from each patient prior to treatment initiation
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Pyrhonen S, Kuitunen T, Nyandoto P, et al. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587-91. [Crossref] [PubMed]
- Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993;72:37-41. [Crossref] [PubMed]
- Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163-8. [Crossref] [PubMed]
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [Crossref] [PubMed]
- Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004;22:2395-403. [Crossref] [PubMed]
- Lee J, Lim T, Uhm JE, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol 2007;18:886-91. [Crossref] [PubMed]
- Takahari D, Boku N, Mizusawa J, et al. Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912. Oncologist 2014;19:358-66. [Crossref] [PubMed]
- Aizawa M, Nagatsuma AK, Kitada K, et al. Evaluation of HER2-based biology in 1,006 cases of gastric cancer in a Japanese population. Gastric Cancer 2014;17:34-42. [Crossref] [PubMed]
- Yano T, Doi T, Ohtsu A, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep 2006;15:65-71. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Janjigian YY, Werner D, Pauligk C, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol 2012;23:2656-62. [Crossref] [PubMed]
- Shitara K, Yatabe Y, Matsuo K, et al. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer 2013;16:261-7. [Crossref] [PubMed]
- Qiu MZ, Li Q, Wang ZQ, et al. HER2-positive patients receiving trastuzumab treatment have a comparable prognosis with HER2-negative advanced gastric cancer patients: a prospective cohort observation. Int J Cancer 2014;134:2468-77. [Crossref] [PubMed]
- Madsen LT. Cancer Prediction Nomograms for Advanced Practitioners in Oncology. J Adv Pract Oncol 2014;5:380-2. [PubMed]
- Giordano A, Egleston BL, Hajage D, et al. Establishment and validation of circulating tumor cell-based prognostic nomograms in first-line metastatic breast cancer patients. Clin Cancer Res 2013;19:1596-602. [Crossref] [PubMed]
- Hirabayashi S, Kosugi S, Isobe Y, et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol 2014;25:1179-84. [Crossref] [PubMed]
- Ashfaq A, Kidwell JT, McGhan LJ, et al. Validation of a gastric cancer nomogram using a cancer registry. J Surg Oncol 2015;112:377-80. [Crossref] [PubMed]
- Kadowaki S, Komori A, Takahari D, et al. Clinical Characteristics Associated with Long-term Survival in Metastatic Gastric Cancer after Systemic Chemotherapy. Asian Pac J Cancer Prev 2015;16:5433-8. [Crossref] [PubMed]
- Shitara K, Matsuo K, Mizota A, et al. Association of fluoropyrimidines, platinum agents, taxanes, and irinotecan in any line of chemotherapy with survival in patients with advanced gastric cancer. Gastric Cancer 2011;14:155-60. [Crossref] [PubMed]
- NCCN clinical practice guidelines in Oncology GC, Ver3. 2016. (accessed Oct 30, 2016). Available online: https://www.nccn.org/professionals/physician_gls/default.aspx
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [Crossref] [PubMed]
- Nguyen CT, Kattan MW. How to tell if a new marker improves prediction. Eur Urol 2011;60:226-8; discussion 228-30. [Crossref] [PubMed]
- Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012;30:3834-40. [Crossref] [PubMed]
- Capanu M, Gönen M. Building a Nomogram for Survey-Weighted Cox Models Using R. J Stat Softw 2015;64:1-17. [Crossref]
- Custodio A, Carmona-Bayonas A, Jimenez-Fonseca P, et al. Nomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumab. Br J Cancer 2017;116:1526-35. [Crossref] [PubMed]
- Sagman U, Feld R, Evans WK, et al. The prognostic significance of pretreatment serum lactate dehydrogenase in patients with small-cell lung cancer. J Clin Oncol 1991;9:954-61. [Crossref] [PubMed]
- Kohne CH, Cunningham D, Di Costanzo F, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol 2002;13:308-17. [Crossref] [PubMed]
- Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2014;32:671-7. [Crossref] [PubMed]


