Aggressive mutation in a familial adenomatous polyposis syndrome family: when phenotype guides clinical surveillance
Background
Familial adenomatous polyps (FAP) is an autosomal dominant genetic condition, caused by mutations in the adenomatous polyposis coli gene (APC) located in chromosome 5q21. Less than 1% of colonic hereditary cancer is explained by a mutation in the APC gene (1,2).
The APC protein has multiple designated segments that make possible its multiple cellular functions and plays a major role in tumor suppression, by antagonizing the WNT signaling pathway; and in preventing cell overgrowth.
Classical FAP has been historically characterized by the presence of more than a hundred adenomatous polyps, distributed in both upper and lower gastrointestinal tract. The variants of FAP are distinguished by the number of polyps or by extra-colonic manifestations (3).
Desmoid tumors (DTs), found in 15% to 20% of FAP patients, being considered as an aggressive form of neurofibromatosis, are defined as locally infiltrating musculo aponeurotic neoplasms derived from connective tissue (4). The growth rate could be colossal. It’s enlargement is frequently the cause of organ compression causing clinical complications and, in some cases even death (5). DTs are classified as: (I) abdominal (anterior abdominal wall); (II) intra-abdominal (mesentery or pelvis, intraperitoneal or retroperitoneal), site of preference, as seen in almost 50% of cases; and (III) extra-abdominal (chest, head and neck region and extremities) (6,7).
Polyps usually present during young adolescence, whereas cancer diagnosis frequently appears in young adulthood. Extra-colonic malignancies are also part of the syndrome, such as: papillary thyroid cancer (2–3%), hepatoblastoma (1–2%), medulloblastoma; (<1%), pancreatic (1%), gastric (1–2%), and duodenal cancer (4–12%) (8). FAP associated-gastric lesions are: fundic gland polyps, gastric adenomas, and gastric cancers.
The main death causes in these patients regarding extra-colonic lesion are as follows: desmoid tumours (9.9%), duodenal cancer (5.6%), and gastric cancer (2.8%) (9).
A genotype-phenotype correlation in FAPs was described for the first time in 1992 (10).
The association between certain extra-colonic manifestations and the locus of the APC mutation, reflects the role that the APC protein plays in different tissues, the age of onset and severity of the disease in those mutations located further from 3' of the APC gene (11). Although a genotype–phenotype correlation was described, an important variability among patients, even among family members, makes us think that the effect of APC-modifying genes and/or environmental factors may influence the expression of the disease.
The aim of this article is to present and analyse a case of a severe, early onset family presenting with lethal extra-colonic manifestations.
Patients and methods
Patients
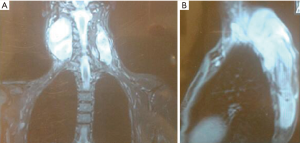
The proband, a female patient (II.7) was diagnosed with a left scapular joint soft tissue DT at the age of 20 (Figure 1). From then onwards, she was operated on and then even received polychemotherapy (PQT), reaching tumor stability evidenced in magnetic resonance imaging (MRI) months later (Figure 2).
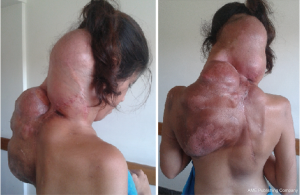
At 21 she developed tumor progression, severe pain with neuropathic component, resistant to pain management and she underwent surgery for tumor mass-reduction, including muscles from the paravertebral leak. Due to a local tumor recurrence, she was once again surgically treated at age 22. The first-line chemotherapy consisted on 4 cycles of liposomal doxorubicin, not showing much progress. For second-line chemotherapy 5 cycles of Crisafeno, also not entirely successful.
At 23 years old, the tumor progression was again confirmed, and a systemic treatment based on liposomal doxorubicin was administered intermittently during two years, when she was re-intervened. As third-line chemotherapy a combination of Methotrexate and Vinblastine was provided.
At 26 treatment shifted to a palliative PQT and radiotherapy (RT), starting with 4 cycles of liposomal doxorubicin, continuing with cyclophosphamide prophylaxis, showing tumor remission. The RT was interrupted due to radiodermatitis and skin infection.
Her fists control endoscopy revealed 22 gastric polyps and colonic mucosae compatible with multiple polyposis [50–100]. Non-malignant features such as supernumerary teeth and head osteomas were also identified. Finally, she was referred to a genetic counselling. At 27, she died due to desmoid tumor progression.
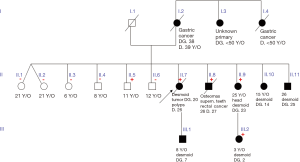
Proband’s and relative’s medical records were gathered from different health care centres and a genealogy was constructed (Figure 3).
Informed consent was previously taken from all consenting adults and minors’ legal tutors when necessary.
For all individuals who chose to be counselled and tested, the Uruguayan Collaborative Group (UCG), committed to register, diagnosis and investigate hereditary cancer syndromes, conformed by a multidisciplinary team of experts, conducted the high risk assessment consults and absorbed the genetic testing financial burden, through a fund raising foundation (www.fundaciongenesis.org.uy).
Methods
DNA from the probands oral mucosa was obtained using commercial Kits. Analysis was made with Next Generation Sequencing (NGS).
Sanger sequencing was offered to all family members willing to be tested.
Results
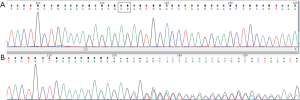
From a family composed by 16 members, ten were tested, three did not adhere to surveillance program and chose not to be tested, and three were dead before the genetic counselling high risk assessment began (two gastric cancer and one rectal cancer victims). Genetic counseling was performed pre and post-test. Using the NGS technology a 2-bp deletion (c.4393_4394delAG) at 3'end of APC gene was detected. Sanger sequencing confirmation was later done (Figures 4,5).
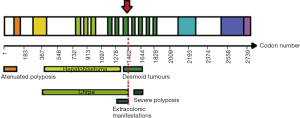
Six individuals presented DTS. The median age for DT diagnosis was 15.2 (±9.2) years, ranging from 2 to 25. Clinical characteristics are described in Table 1.
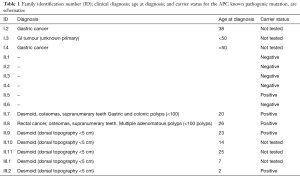
Full table
There is a very clear anticipation phenomenon pattern, given by successive earliest ages of diagnosis for the third generation (Table 1).
In five patients, a pathogenic, heterozygote mutation, located in the APC gene was detected. Four of the individuals were carriers of DTs, the remaining mutation carrier is an asymptomatic 11 years old boy. None of young family members has undergone endoscopic examination.
Discussion
Here we presented a case that follows an autosomal dominant transmission pattern. It was caused by an APC germline AG deletion at 1,465 codons with a penetrance near 100% for DTs, and a very clear anticipation phenomenon. The presence of a relatively small number of colonic polyps found in two out of five mutation carriers (II.7 and II.8), indicates that the codon 1,465 frameshift mutation is incompletely penetrant in the GI tract, which is evidenced by a late-onset and attenuated forms of polyposis.
DTs are benign extra-intestinal lesions associated with colonic polyposis, which can turn into a serious or mortal complication for FAP individuals.
To date, there is not enough evidence for a clear genotype-phenotype correlation to DTs severity and incidence. They are mostly associated with mutations between codons 1,250 and 1,400 of the APC gene that mainly end in a truncated protein (15,16). According to Caspari et al., mutations located between codon 1,445 and 1,578 result in DTs, among other manifestations (osteomas, epidermoid cysts, and polyps of the upper GI tract) (17). The phenotypic variability may lay on the two-hit mechanism.
The mutation causes a premature translation stop, therefore, a shortened APC protein: p.S1465WfsX3. This mutation has been previously reported (18,19).
In this family group, the mutation in codon 1,465 of APC gene is associated to a phenotype consisting in blossoming DTs located in dorsal region, with relatively low polyposis burden. Although the mechanisms of this variability must still be elucidated, it can be possible that a different modifier gene favored the ‘second hit’ in mesenchymal cells (in II.7), or in gastric cells (II.8) producing the typical GI or desmoids tumors, respectively (20,21).
The National Comprehensive Cancer Network emphasises that there is not enough data to support specific screening and treatment for DTs [hormone therapies, no steroidal anti-inflammatory drugs (NSAIDs), targeted therapies, and traditional cytotoxic chemotherapies]. The recommendations encourage physicians to make individualized surveillance to account for genotype, phenotype and personal considerations (22). The absence of more precise guidelines has motivated our group (UCG) to state some considerations for a more adequate patient management.
Once a patient is clinically diagnosed as FAP, a genetic study must be performed since the genotype helps guide surveillance. Although there is some heterogeneity in clinical manifestations of FAP, even among family members with the same APC gene mutation, the correlation between the site of the mutation within the gene and the clinical manifestations of the disease is useful for the clinical management of affected patients (23,24). A multidisciplinary approach with different individualized treatments should be extremely beneficial. Patients with desmoids located in the head and neck region that could became in life-threatening complications need more aggressive and prompt treatment (25). RT in combination with surgery results in better local control than surgery alone, even in recurrent DT (26). Evidence for the efficacy of treatments is limited because it’s based on small, non-controlled studies. The small number of cases in even major tumor centers has limited the ability to better study patients with DTs (27).
In conclusion, for overall better management and outcome, it is imperative to offer genetic counseling risk assessment and tailor surveillance for each family member, according to: risk profile, age, comorbidities, mutation site and access to healthcare. It should also be performed in specialized centers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Church J. Familial Adenomatous polyposis. Surg Oncol Clin N Am 2009;18:585-98. [Crossref] [PubMed]
- Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genet Med 2010;12:726-35. [Crossref] [PubMed]
- Ellis CN. ColonicAdenomatousPolyposis Syndromes: Clinical Management. Clin Colon Rectal Surg 2008;21:256-62. [Crossref] [PubMed]
- Li L, Jensen J, Szabo S, et al. Recurrent giant cranial desmoidtumor in a 3-year-old boy with familial adenomatous polyposis requiring bifrontoparietalcranioplasty: case report. J Neurosurg Pediatr 2016;25:703-7. [Crossref] [PubMed]
- Honeyman JN, La Quaglia MP. DesmoidTumors in the Pediatric Population. Cancers 2012;4:295-306. [Crossref] [PubMed]
- Lewis JJ, Boland PJ, Leung DH, et al. The enigma of desmoid tumours. Ann Surg 1999;229:866-72. [Crossref] [PubMed]
- Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg 1996;83:1494-504. [Crossref] [PubMed]
- Leoz ML, Carballal S, Moreira L, et al. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl Clin Genet 2015;8:95-107. [PubMed]
- Iwama T, Tamura K, Morita T, et al. A clinical overview of familial adenomatous polyposis derived from the database of the Polyposis Registry of Japan. Int J Clin Oncol 2004;9:308-16. [Crossref] [PubMed]
- Nagase H, Miyoshi Y, Horii A, et al. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res 1992;52:4055-7. [PubMed]
- Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): A review of the literature. Crit Rev Oncol Hematol 2007;61:153-61. [Crossref] [PubMed]
- Groen EJ, Roos A., Muntinghe FL, Enting RH, et al. Extra-Intestinal Manifestations of Familial Adenomatous Polyposis. Ann Surg Oncol 2008;15:2439-50. [Crossref] [PubMed]
- Wallis YL, Morton DG, McKeown CM, et al. Molecular analysis of the APC gene in 205 families: Extended genotype-phenotype correlations in FAP and evidence for the role of APC amino acid changes in colorectal cancer predisposition. J Med Genet 1999;36:14-20. [PubMed]
- Bertario L, Russo A, Sala P, et al. Hereditary Colorectal Tumor Registry. Multiple approaches to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J Clin Oncol 2003;21:1698-07. [Crossref] [PubMed]
- Couture J, Mitri A, Lagace R, et al. A germline mutation at the extreme 3' end of the APC gene results in a severe desmoid phenotype and is associated with overexpression of beta-catenin in the desmoidtumor. Clin Genet 2000;57:205-12. [Crossref] [PubMed]
- Wachsmannova-Matelova L, Stevurkova V, Adamcikova Z, et al. Different phenotype manifestation of familial adenomatous polyposis in families with APC mutation at codon 1309. Neoplasma 2009;56:486-9. [Crossref] [PubMed]
- Caspari R, Olschwang S, Friedl W, et al. Familial adenomatous polyposis: desmoid tumours and lack of ophthalmic lesions (CHRPE) associated with APC mutations beyond codon 1444. Hum Mol Genet 1995;4:337-40. [Crossref] [PubMed]
- Martin-Denavit T, Duthel S, Giraud S, et al. Phenotype variability of two FAP families with an identical APC germline mutation at codon 1465: a potential modifier effect? Clin Genet 2001;60:125-31. [Crossref] [PubMed]
- Miyaki M, Konishi M, Kikuchi-Yanoshita R, et al. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer research 1994;54:3011-20. [PubMed]
- Dobbie Z, Heinimann K, Bishop DT, et al. Identification of a modifier gene locus on chromosome 1p35-36 in familial adenomatous polyposis. Hum Genet 1997;99:653-7. [Crossref] [PubMed]
- Crabtree MD, Fletcher C, Churchman M, et al. Analysis of candidate modifier loci for the severity of colonic familial adenomatous polyposis, with evidence for the importance of the N-acetyl transferases. Gut 2004;53:271-6. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Colorectal High Risk Guidelines. Version 2. 2016.
- Ghert M, Yao X, Corbett T, et al. Treatment and follow-up strategies in desmoidtumours: a practice guideline. Curr Oncol 2014;21:e642-9. [Crossref] [PubMed]
- Syngal S, Brand R, Church J, et al. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol 2015;110:223-62. [Crossref] [PubMed]
- Kasper B, Ströbel P, Hohenberger P. Desmoid Tumors: Clinical Features and Treatment Options for Advanced Disease. Oncologist 2011;16:682-93. [Crossref] [PubMed]
- Nuyttens JJ, Rust PF, Thomas CR Jr, et al. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors. A comparative review of 22 articles. Cancer 2000;88:1517-23. [Crossref] [PubMed]
- Rampone B, Pedrazzani C, Marrell D, et al. Updates on abdominal desmoidtumors. World J Gastroenterol 2007;13:5985-8. [Crossref] [PubMed]


