Current survival and treatment trends for surgically resected intrahepatic cholangiocarcinoma in the United States
Introduction
Although rare, the incidence of intrahepatic cholangiocarcinoma (ICC) has increased over the last several decades (1). ICC represents the second most common primary liver malignancy and has an insidious clinical course. ICC is commonly diagnosed at advanced stages with intra- and extra-hepatic metastases and peritoneal carcinomatosis precluding surgical resection. Surgical resection remains the only option for cure. However, in patients who undergo resection, the median survival is only 20–40 months with 5-year survival rates of 20–40% (2-6), highlighting the inadequacy of current surgical resection as the sole treatment modality.
The inclusion of a lymphadenectomy (LAD) as a component of surgical resection is debated. It is well accepted that lymph node (LN) metastasis at the time of the index operation is a poor prognostic factor for ICC (2,4,7-12). Despite this, the role of LAD remains ill defined. While routine LN dissection does not show a survival advantage, proponents of LAD believe it provides an accurate nodal staging, important prognostic information, and assists in the decision making regarding adjuvant treatment (13,14). The recently published 8th American Joint Committee on Cancer (AJCC) staging system made several modifications in order to more appropriately stratify prognosis, which included a new recommendation for the removal of at least six LNs at the time of surgical resection (15). Previously, there was no standardized recommendation on the extent of LAD in the United States. The current National Comprehensive Cancer Network (NCCN) guidelines for ICC state that “a portal lymphadenectomy is reasonable as this provides relevant staging information” (16). The literature reports rates of LAD at the time of index surgery range from 26% to 67% (4,6,17,18). Despite the important prognostic information that nodal status provides, as well as new recommendations to perform a LN dissection, it is unclear if surgeons are currently performing adequate LAD at the time of hepatic resection for localized ICC.
Due to the rarity of the disease and lack of level 1 evidence to guide treatment, there is no consensus on the appropriate neoadjuvant or adjuvant treatments for ICC. Current NCCN guidelines recommend the addition of adjuvant treatment for patients with R1/R2 disease and positive nodes (16).
Despite the creation of a specific ICC staging system by the AJCC in 2009 and its recent modifications, it is unclear whether clinical practice or outcomes have changed. Given this, our objectives were to evaluate the current practice trends, clinical outcomes and overall survival (OS) for patients with surgically resected ICC, with specific regard for LAD and chemotherapy.
Methods
Data and patients
We used an augmented version of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, including both radiation and chemotherapy data, to identify subjects diagnosed with ICC from 2000 through 2014. The SEER registries provide cancer surveillance for 18 geographic areas and represents 28% of the United States population. The registry collects both patient and tumor characteristics, including age at diagnosis, race, patient sex, primary tumor site, histologic subtype, tumor stage (T stage), tumor size, LN status, tumor grade, LN evaluation, diagnostic confirmation, type of surgery, vital status, and cause of death. We identified patients ≥18 years old who were diagnosed with ICC using the World Health Organization’s International Classification of Diseases, 3rd revision (ICD-3) codes (Table S1). We excluded records of patients with distant- or un-staged tumors based on historical stage. As only de-identified patient data was used, this study was exempt from review by the Institutional Review Board of the University of Minnesota.

Full table
Statistical analysis
We categorized patients based on number of LNs assessed at surgery into 0 LNs assessed, 1–5 LNs assessed, and 6 or greater LNs assessed and compared patterns of LN assessment over the 14-year study period using Cochran-Armitage test for trend. After assessing unadjusted relationships, we used multivariable logistic regression to assess factors associated with any LAD and adequate LAD (≥6 LNs). Factors assessed in our regression models included age, gender, race, year of diagnosis, tumor grade, and T stage. Next, we assessed current utilization trends of chemotherapy over the 14-year time period using Cochrane-Armitage test for trend. Multivariable logistic regression was also used to evaluate factors associated with utilization of chemotherapy. The model included age, gender, race, year of diagnosis, grade, nodes evaluated, LN status, and T stage.
To analyze OS we used the Kaplan-Meier method and Cox proportional hazard models. Our Cox proportional hazards models included age at diagnosis, race, tumor size, tumor grade, LN status, and use of chemotherapy. In all models, we performed sensitivity analyses to ensure that the observed effects were not a product of coding classifications such as the use of alternative node evaluation groupings and removal of non-significant factors. Results were considered statistically significant only for a P value ≤0.05 and a 95% confidence interval (CI). For all statistical analyses, we used SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient population
We identified 1,263 patients who underwent surgical resection for ICC (Table 1). Most patients were older than 60 years of age (65%) and white (79%). Most tumors were grade II (41%), T1 tumors based on TNM staging criteria (41%), and were 5 cm or larger (45%). The majority of patients did not receive either chemotherapy or radiation therapy (58%).
Full table
LAD at the time of resection
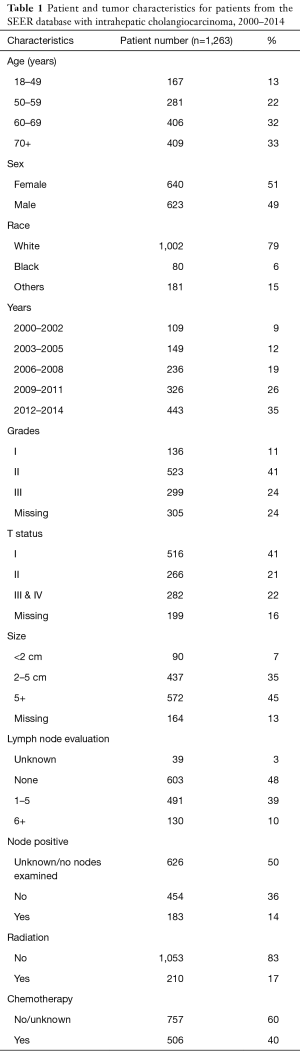
We found that 621 patients (49%) underwent LAD, while 603 patients (48%) had no LAD at the time of surgical resection. Based on the new AJCC guidelines for adequate LAD, 39% underwent an inadequate LAD (1–5 nodes assessed), and only 10% had an adequate LAD (Table 1). Although a large proportion of patients did not receive AJCC recommended LAD, the rates of 6 or greater LN assessed significantly increased over time (P<0.05) (Figure 1A).
Factors associated with LAD
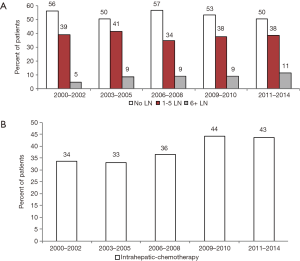
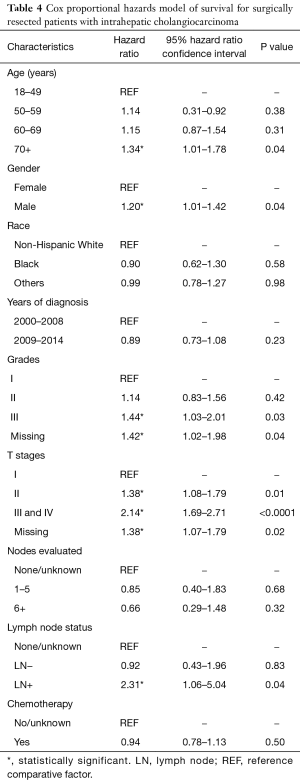
Multivariable analysis was performed to evaluate factors associated with performance of LAD (Table 2). The factors associated with a significantly decreased likelihood of LAD were older age and race other than white or black (Table 2). Higher T stage was associated with a higher likelihood of LAD (Table 2). With respect to the performance of an adequate LAD, patients with older age, male gender, and black race were less likely to undergo an adequate LAD. Increasing T stage was associated with an increased likelihood of adequate LAD (Table 2). Diagnosis of ICC during or after 2009, the year the first ICC-specific AJCC staging system was published, was not associated with any or adequate LAD.
Full table
Utilization of chemotherapy and factors associated with chemotherapy use
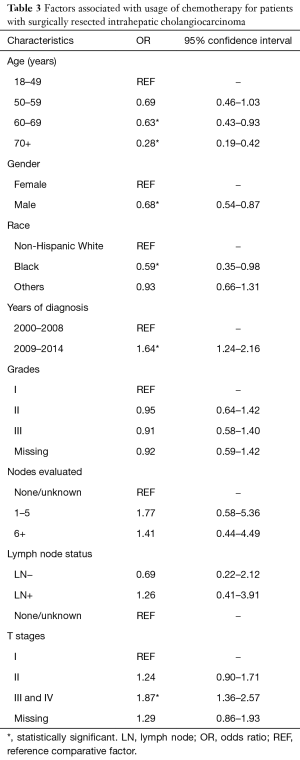
There was a significant increase in the usage of chemotherapy in patients with ICC over time (P<0.05) (Figure 1B). The percentage of surgical patients undergoing chemotherapy treatment before 2009 ranged from 33% to 36% and increased to 43–44% after 2009. Multivariable regression analysis was performed to identify the factors associated with chemotherapy use and is shown in Table 3. Factors associated with a greater likelihood of receipt of chemotherapy included year of diagnosis in 2009–2014 and T stage. Factors associated with a lower likelihood of treatment with chemotherapy included increasing age, male gender, and black race. LN evaluation and LN status (positive or negative) compared to unknown was not predictive of chemotherapy use (Table 3). Of note, when LN status is excluded, the performance of any LAD increases the likelihood of receipt of chemotherapy (OR, 1.40; 95% CI, 1.09–1.79, data not shown in table). On univariate analysis, LN-positive patients were more likely have chemotherapy treatment compared to LN-negative patients (OR, 1.82; 95% CI, 1.24–2.64, data not shown).
Full table
ICC survival
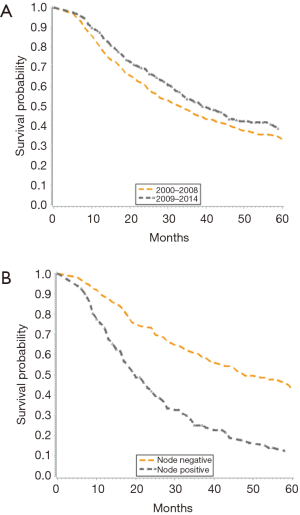
When stratified by time period (2000–2008 vs. 2009–2014), there was an improvement in median survival from 33 to 39 months (Figure 2A). Overall, unadjusted Kaplan-Meier mortality estimates showed significantly lower OS in the earlier time period. Further, nodal status was shown to significantly impact OS, as node negative patients had a median survival of 52 months compared to 20 months for those with node positive disease (Figure 2B).
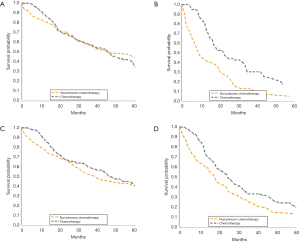
There was no significant survival benefit to the addition of chemotherapy, with a median survival of 37 months for patients who did and did not receive chemotherapy (data not shown). When evaluated by node status, node negative patients had no added benefit to chemotherapy, with median survival of 46 months in both groups (Figure 3A). Node positive patients derived a significant benefit from chemotherapy, with median survival of 9 months in those not treated with chemotherapy and 23 months in those treated with chemotherapy (Figure 3B). In patients with lower T stages (T1/T2), chemotherapy correlated with an 8-month increase in OS from 39 months without chemotherapy to 47 months with chemotherapy (Figure 3C). There was also a survival benefit to chemotherapy in patients with advanced T stages (T3/T4). Median survival for patients with T3/T4 tumors treated with chemotherapy was 25 vs. 18 months in those treated without chemotherapy (Figure 3D). When we adjusted for all other patient factors, the factors associated with a greater hazard of death included age greater than 70 years, male gender, tumor grade III, increasing T stage and LN positive status (Table 4).
Full table
Discussion
Due to its rarity, there are very few large-scale studies from which to draw meaningful conclusions about appropriate management for patients with ICC. Outcomes for ICC have historically been poor and there is limited evidence to suggest that modern treatment has significantly improved outcomes. Overall 5-year survival for patients with ICC was estimated at less than 5% from 1975 to 1999 (19). However, more recent studies show that for patients with surgically resectable disease, 5-year OS may approach 20–40% with median survival nearing 20–40 months (2-6). In this retrospective review of a national database over a 15-year time period, we found: (I) OS for patients with resected ICC improved; (II) chemotherapy utilization increased; and (III) surgical resection without LAD remained high, >50%. There is no doubt that some patients are being under staged and potentially undertreated with adjuvant therapy.
In the present study, we demonstrated an improvement in OS for patients diagnosed with ICC after the implementation of the initial ICC-specific AJCC staging system in 2009, compared to patients diagnosed prior. A previous review of the SEER database found that median OS for patients with resected ICC from 1992 to 2002 was 22 months (20). Taken together, this demonstrates that continued advances in surgical and medical therapy are slowly making improvements in OS for ICC patients.
The source of the improvement in outcomes over this time period is unclear, but may correlate with the increased utilization of chemotherapy over time. This may in part contribute to the survival improvement seen; however, chemotherapy use alone was not found to improve OS on multivariable analysis. In our study, we found a survival benefit to chemotherapy in patients with node positive disease and advanced T stages. This is in accordance with previously published data that chemotherapy may benefit high-risk patient subsets (7,21-23), and indicates that better patient selection for adjuvant therapy may lead to improved survival outcomes. A previous study using the NCDB database found that adjuvant chemotherapy was only associated with a survival benefit in patients with node positive disease, advanced T stage tumors (T3 or T4) and incomplete resections (R1 or R2) (7). Another survey of the NCDB affirmed that the patients who benefited from chemotherapy had either positive LNs or positive margins after resection (21). Single institution series have also shown an OS benefit to the use of adjuvant treatment in biliary tract cancers in patients with node positive disease or an R1 resection (22). A systematic review which identified twenty studies involving greater than 6,000 patients with biliary tract cancers found a non-significant improvement in OS with adjuvant treatment; however, patients with high-risk features, such as margin and node positive disease, were found to derive a significant benefit to adjuvant therapy (23). While there appears to be a survival advantage for chemotherapy in certain subsets of patients (incomplete resections, advanced T stage and node positive disease), there are no results from a prospective randomized trial to guide neoadjuvant or adjuvant treatment practices. The confusion is further exemplified by a systematic review which had previously found no benefit for adjuvant treatments in patients with ICC (6). Currently, there is an ongoing stage III randomized clinical trial of adjuvant chemotherapy in surgically resected biliary tract cancer patients, which will hopefully provide further guidance for chemotherapy use (24).
With respect to surgical management of ICC, there is ongoing debate as to the extent, if any, of LAD. The recommendation for LAD was added to the 8th edition of the AJCC staging system, despite the evidence that the role of LAD for anything other than prognostication remains uncertain. LN positivity has repeatedly been shown to be a negative prognostic factor in ICC (2,4,7-12,25-27). We found that LAD occurred in 49% of patients which is comparable to previously reported rates from 25% to 67% (2,4,6,17,18). In the current study, 29% of patients who underwent LAD (removal of any number of nodes) were found to have metastatic disease while other studies have reported rates from 20% to 62% (21,28,29). Young age and advanced T stage were associated with LAD, suggesting that the current national practice is selective use of LAD. Perhaps, the node positivity rate for patients who currently do not undergo LAD would be less than the node positivity rates listed above. Nonetheless, node positive disease has a strong negative prognostic implication, as confirmed in this study, and patients undergoing surgical resection should expect to have appropriate nodal staging.
Accurately defining LN status could improve receipt of adjuvant treatment and outcomes. One study showed that at 18 months of follow-up, patients who did not undergo LAD at the time of resection for ICC (Nx patients), had worse survival than patients who were N0 stage after LAD, although the survival advantage did not persist after 18 months (29). Similarly, at 18 months of follow-up, the disease-free survival for patients classified as Nx was comparable to patients with N1 disease; however, with longer term follow-up, the patients with nodal metastasis had a worse outcome (29).
The newest version of the AJCC is the first to recommend that LAD include retrieval of 6 LNs (15). In our study, we found that more than 50% of patients did not undergo any LAD at all, and only 10% of patients received an adequate LAD. While extent of LAD may not entirely impact survival (30), another study showed an improvement in OS for N0 patients who had higher numbers of LN harvested (18). Even if LAD itself does not improve survival, adequate nodal evaluation is necessary for accurate staging and may inform treatment decisions. In the present study, we found that when LN status was excluded from the multivariable logistic regression model, patients who did not undergo LAD were less likely to receive chemotherapy. This suggests that patients who do not undergo LAD are less likely to receive adjuvant chemotherapy, which may result in an OS decrement. Nonetheless, it is clear that current surgical practices in the United States do not adhere to the recent AJCC recommendations for an adequate LAD. Furthermore, since there are no randomized trials to guide treatment for ICC, many recommendations for management of patients with ICC rely on retrospective studies of population based databases. By including inadequately staged patients these recommendations may contain significant bias.
While we provide a large retrospective review of current practice trends in the United States using the SEER database, we acknowledge that there are several potential limitations to this design. This study is an analysis of a retrospective database and, as such, may introduce selection bias. Furthermore, while the SEER database contains a number of useful clinical parameters, there are several important prognostic factors which are missing, such as margin status and comorbidities. Additionally, chemotherapy data from the SEER database is also flawed as it is distributed as a binary variable “yes” or “no/unknown”, making it impossible to accurately distinguish between “no” and “unknown” treatment. Further, the SEER database also lacks details on the specific chemotherapeutic strategy used for treatment, specifically if it is adjuvant or neoadjuvant; however, given limited use of neoadjuvant chemotherapy in this population during the time course studied, it is unlikely that this would impact a large subset of patients. Moreover, we found that the use of chemotherapy observed corresponds with findings in the literature (7,21,29). Finally, SEER cancer registries do not collect information on patient prognostic factors such as margin status, patient comorbidities, patients’ performance status, nutritional status, and surgeon and hospital volume.
In conclusion, this retrospective review of the large SEER database demonstrates that OS for ICC is improving with time. The negative prognostic value of LN metastasis and increased focus on LN evaluation notwithstanding, more than 50% of patients who underwent resection for ICC did not receive a LAD. As chemotherapy has been associated with improved survival in patients with high-risk features and LN metastases, LN evaluation should become a routine component of surgery for ICC for prognostication and stratification purposes, which will inform adjuvant therapy treatment decisions.
Acknowledgements
The authors would like to thank the Surgical Oncology Department at the University of Minnesota for their helpful insight and discussion when preparing this manuscript. This research was in part funded by the University of Minnesota Department of Surgery Cancer Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As only de-identified patient data was used, this study was exempt from review by the Institutional Review Board of the University of Minnesota.
References
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg 2009;33:1247-54. [Crossref] [PubMed]
- Ribero D, Pinna AD, Guglielmi A, et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg 2012;147:1107-13. [Crossref] [PubMed]
- Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001;193:384-91. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Miura JT, Johnston FM, Tsai S, et al. Chemotherapy for Surgically Resected Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:3716-23. [Crossref] [PubMed]
- Kim Y, Moris DP, Zhang XF, et al. Evaluation of the 8th edition American Joint Commission on Cancer (AJCC) staging system for patients with intrahepatic cholangiocarcinoma: A surveillance, epidemiology, and end results (SEER) analysis. J Surg Oncol 2017;116:643-50.
- Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, et al. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: a population-based study using the National Cancer Institute SEER database. HPB (Oxford) 2011;13:612-20. [Crossref] [PubMed]
- Uchiyama K, Yamamoto M, Yamaue H, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2011;18:443-52. [Crossref] [PubMed]
- Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 2014;149:432-8. [Crossref] [PubMed]
- Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048-56. [Crossref] [PubMed]
- Kim DH, Choi DW, Choi SH, et al. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery 2015;157:666-75. [Crossref] [PubMed]
- Amini N, Ejaz A, Spolverato G, et al. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg 2014;18:2136-48. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th edition. New York, NY: Springer, 2016.
- Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers (Version 3.2017). National Comprehensive Cancer Network 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed 9/4/2017 2018
- Adachi T, Eguchi S. Lymph node dissection for intrahepatic cholangiocarcinoma: a critical review of the literature to date. J Hepatobiliary Pancreat Sci 2014;21:162-8. [Crossref] [PubMed]
- Bagante F, Spolverato G, Weiss M, et al. Assessment of the Lymph Node Status in Patients Undergoing Liver Resection for Intrahepatic Cholangiocarcinoma: the New Eighth Edition AJCC Staging System. J Gastrointest Surg 2018;22:52-9.
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [Crossref] [PubMed]
- Nathan H, Pawlik TM, Wolfgang CL, et al. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 2007;11:1488-96; discussion 1496-7. [Crossref] [PubMed]
- Sur MD, In H, Sharpe SM, et al. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:2209-17. [Crossref] [PubMed]
- McNamara MG, Walter T, Horgan AM, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol 2015;38:382-7. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [Crossref] [PubMed]
- Yedibela S, Demir R, Zhang W, et al. Surgical treatment of mass-forming intrahepatic cholangiocarcinoma: an 11-year Western single-center experience in 107 patients. Ann Surg Oncol 2009;16:404-12. [Crossref] [PubMed]
- Marubashi S, Gotoh K, Takahashi H, et al. Prediction of the postoperative prognosis of intrahepatic cholangiocarcinoma (ICC): importance of preoperatively- determined anatomic invasion level and number of tumors. Dig Dis Sci 2014;59:201-13. [Crossref] [PubMed]
- Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005;29:728-33. [Crossref] [PubMed]
- Li DY, Zhang HB, Yang N, et al. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol 2013;19:9084-91. [Crossref] [PubMed]
- Bagante F, Gani F, Spolverato G, et al. Intrahepatic Cholangiocarcinoma: Prognosis of Patients Who Did Not Undergo Lymphadenectomy. J Am Coll Surg 2015;221:1031-40.e1-4.
- Vitale A, Moustafa M, Spolverato G, et al. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2016;113:685-91. [Crossref] [PubMed]


