Utilization of neoadjuvant intensity-modulated radiation therapy and proton beam therapy for esophageal cancer in the United States
Introduction
Esophageal cancer (EC) is a major cause of not only oncologic mortality, but also treatment-related morbidity. This is especially apparent following trimodality therapy, which is now the standard of care for locally advanced EC (1). In continual efforts to reduce therapy-related toxicities, use of advanced radiotherapy (RT) techniques (ARTs), namely intensity-modulated RT (IMRT) and proton beam therapy (PBT), have come to the forefront of management of EC—often without level I evidence supporting their utility (2-9).
However, virtually all randomized EC trials have utilized either two- or three-dimensional conformal RT (3DCRT) techniques (1,10); in the absence of a randomized comparison, the role of ARTs in EC is uncertain. The National Comprehensive Cancer Network (11) recently revised a statement on RT technique by eliminating the phrase “3-D treatment planning is strongly encouraged” and replaced it with “IMRT or PBT is appropriate in clinical settings where reduction in dose to organs at risk…is required that cannot be achieved by 3-D techniques.”
Although there are substantial drawbacks with ARTs, including the often prohibitive cost and subsequent lack of insurance coverage, there are multiple theoretical (yet unproven) benefits that may be noteworthy in EC. First, treatment in the neoadjuvant setting poses intra- and post-procedural risks; extensive data from MD Anderson Cancer Center have observed fewer postoperative complications following neoadjuvant ART-based therapy (12-15). Next, as survivorship following therapy for EC rises, RT-induced toxicities (e.g., cardiopulmonary) may become significant, as shown for multiple neoplasms (16-18); thus, utilizing ARTs to protect against these late complications may be of benefit (19-24).
Owing to the high controversy surrounding these technologies, evaluating national practice patterns and trends is essential. This is the first study to date evaluating utilization of ARTs versus 3DCRT as part of neoadjuvant CRT for locally advanced EC in the United States. These results have implications for ART utilization going forward as well as insurance coverage by payers.
Methods
This investigation analyzed the National Cancer Data Base (NCDB), which is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which consists of de-identified information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the US population (25-32). The NCDB contains information not included in the Surveillance, Epidemiology, and End Results database, including details regarding use of systemic therapy and radiation dose. The data used in the study were derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
The most recently released NCDB dataset corresponded to the years 2004–2013. Inclusion criteria for this study involved patients age ≥18 with newly-diagnosed cT1b-T4a N0/N+ M0 EC comprising histologic codes of adenocarcinoma (International Classification of Disease for Oncology (ICD-O-3) codes 8140, 8141, 8143, 8144, 8145, 8147, 8255, 8260, 8310, 8340, 8480, 8481) or squamous cell carcinoma (ICD-O-3 codes 8052, 8053, 8070, 8071, 8072, 8073, 8074, 8075, 8076, 8078, 8083, 8084, 8560). For inclusion, patients required histological diagnostic confirmation and receipt of neoadjuvant CRT followed by partial or complete esophagectomy (surgical procedure of the primary site codes 30, 40, 50–55, 80). Since the purpose of the study was to compare the effect of radiation technique, inclusion criteria included the presence of a record of RT technique. Patients receiving either IMRT or PBT were included in the ART cohort, and were compared to patients receiving 3DCRT. In order for inclusion in the study, patients required a radiation dose of at least 35 Gy per published trials (33). The use of concurrent therapy was defined as receipt of chemotherapy within 21 days of RT. Using a classification scheme from other published studies utilizing the NCDB, an academic facility was an institution with both an accession of more than 500 newly diagnosed cancer cases per year and one that provided postgraduate medical education in at least four program areas, including internal medicine and general surgery (34). All other facilities, including Comprehensive Community Cancer Programs, Community Cancer Programs, and Integrated Network Programs, were categorized as non-academic, as none of these institutions require graduate medical education.
Information collected on each patient broadly included demographic data, comorbidity information, clinicopathological tumor parameters, and treatment facility characteristics. All statistical tests were two-sided, with a threshold of P<0.05 for statistical significance, and were performed using Stata (version 14, College Station, TX, USA). Fisher’s exact or χ2 test analyzed categorical proportions between groups in the non-parametric and parametric settings, respectively. The primary goal herein was to evaluate temporal trends and predictors of ART use. Multivariable logistic regression modeling was utilized to determine characteristics that were predictive for receipt of ART. Survival was not expected to show differences between groups and thus was performed only secondarily. The Kaplan-Meier method was used for survival analysis, and comparisons between the ART and 3DCRT groups were performed with the log-rank test. Overall survival (OS) was defined as the interval between the date of diagnosis and the date of death or last contact. Univariate analysis was performed to determine which factors were associated with OS, and subsequently Cox multivariate analysis was performed including variables that were either significant or showed a strong trend to statistical significance on univariate analysis. The proportional hazards assumption was checked graphically using log-log plots. Patients with unknown or not recorded values for income, insurance status, and treatment facility type were excluded from the multivariate logistic regression modeling and the Cox proportional hazards analysis due to their low absolute numbers and lack clinical significance.
Results
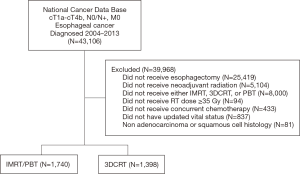
A complete flow diagram of patient selection is provided in Figure 1; 3,138 patients met study criteria. Of these, 1,398 (45%) were treated with 3DCRT, and 1,740 (55%) with ARTs. In the ART cohort, 18 (1%) received PBT and 1,722 (99%) IMRT. Table 1 displays clinical characteristics of the analyzed patients. Of note, most patients had adenocarcinomas located in the distal esophagus and locally advanced disease.
Full table
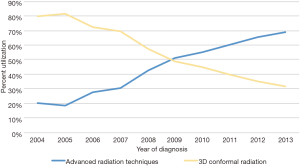
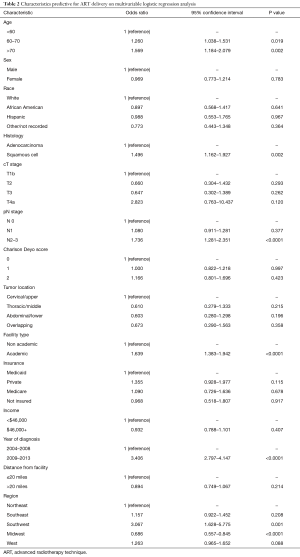
The primary objective of this study was to evaluate temporal trends and predictors of ART delivery. Figure 2 displays that utilization of ARTs is steadily rising in the United States, from 20% in 2004 to 69% in 2013, which corresponds with a progressive drop in 3DCRT. Multivariable logistic regression analysis was performed to evaluate factors independently associated with receiving ARTs (Table 2). ARTs were more often delivered to patients with advancing age, squamous cell histology, N2+ disease, and at academic centers (P<0.05 for all). There were also regional differences: as compared to the Northeast US, Southwestern (P=0.001) and Western (trend, P=0.088) regions were more likely to deliver ART; the Midwest was less likely to do so (P<0.001). Corroborating the results of Figure 2, there was a powerful and independent influence of time period on ART administration, with more recent years associated with over a threefold higher likelihood of ART delivery (odds ratio 3.41, 95% confidence interval 2.80–4.15, P<0.001).
Full table
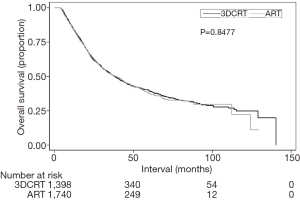
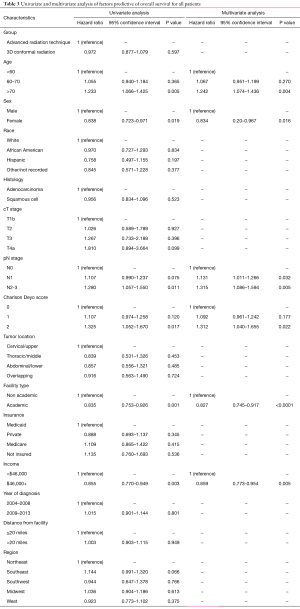
Secondary analyses of the dataset included OS analysis and evaluation of postoperative events. Median follow-up was 25.4 months (interquartile range (IQR), 15.3–42.3 months). Kaplan-Meier estimates of OS between groups are illustrated in Figure 3; as expected, there were no differences in OS between the ART and 3DCRT cohorts (38.8 vs. 38.2 months, P=0.8477). Multivariate Cox proportional hazards modeling examining independent predictors of OS is displayed in Table 3. There were several factors associated with poorer OS: age >70, male gender, node-positive disease, Charlson Deyo comorbidity index of 2, lower income, and treatment at a community facility (P<0.05 for all). Lastly, as coded by the NCDB, there were no differences between the respective groups in terms of 30-day mortality (3.7% vs. 3.4%, P=0.575), 90-day mortality (7.8% vs. 6.8%, P=0.303), average postoperative hospitalization (12.2 vs. 12.2 days, P=0.955), or 30-day readmission rates (6.0% vs. 5.9%, P=0.963).
Full table
Discussion
Although the value of trimodality therapy in EC is now more clearly defined, there remains substantial controversy regarding optimal RT technique in this setting. There are numerous findings and reflections from this analysis of a contemporary national database, the largest of its kind to date. Utilization of ARTs (IMRT in the vast majority) is steadily rising in the United States, and 3DCRT is now used in a minority of patients. ART use is impacted by not only age and disease factors, but also regional and facility differences. As expected, there was no influence of technique on OS, and data did not display differences in postoperative events.
It is relatively clearer that ART utilization is increasing among practitioners in the United States. The results of multivariable logistic regression analysis suggest that ARTs were more often delivered for patients at higher risk of toxicities, including those that were older and with nodal positivity. Regional differences signal that advanced technologies often permeate certain areas of the United States before others. It is also very important from a medico-economic perspective that ART delivery was not impacted by socioeconomic or insurance-related factors. Although this could be a result of payers being open to ARTs for management of EC despite the lack of proven benefit in cost-effectiveness, there are many insurers who may not routinely cover ARTs, to which the results of this investigation may be substantially useful.
In addition, academic centers—largely the drivers of advancements in radiation oncology—were also more likely to deliver ARTs. The independent association between treatment at an academic facility and OS on Cox multivariate analysis has far-reaching implications on patient counseling and management by both oncologists and referring providers. These findings are in concord with data from other neoplasms demonstrating improved outcomes at academic and/or high-volume facilities (35). There are several potential reasons for this, not limited to greater multimodality coordination, streamlined diagnostic processes, technical expertise of a major surgical procedure, ancillary support staff for close clinical monitoring and clinical support such as treatments offered by speech therapy and nutrition services, greater access to rehabilitative services in academic centers, and potentially the availability of salvage therapies (or clinical trials). Nevertheless, this finding may impact any case of locally advanced EC and could warrant revisions in patterns of patient education.
The primary objective of this pattern of care study was not to evaluate OS, as it was predictable that no OS differences existed between groups. The goal of IMRT is primarily for toxicity reduction and not outcome improvement. Indeed, retrospective studies comparing outcomes for patients with EC treated with either IMRT or 3DCRT have failed to show any difference in OS, though they have demonstrated decreased rate of toxicity with the use of IMRT (36-38). However, although PBT patients comprised a minority of the ART cohort herein (and thus could not be reliably compared with the IMRT or 3DCRT cohorts), an ongoing randomized trial of PBT versus IMRT is evaluating both toxicity and progression-free (but not overall) survival primary endpoints (39). Next, the finding of no differences in postoperative events between ART and 3DCRT may seemingly conflict with aforementioned data (9-12), but a major area of caution from this investigation is the patients without a coded RT technique had to be excluded. This amounted to over double the patients that were included in this study. Additionally, definitions of postoperative events and complications are undoubtedly different from the NCDB and the aforementioned published work. Hence, conclusions from this paper must be tempered and do not necessarily point to the notion that 3DCRT yields equivalent postoperative events as ARTs.
There are several shortcomings of this investigation. First, in addition to retrospective selection biases, issues regarding the NCDB’s lack of coding RT technique have been discussed above. Second, the NCDB does not keep track of several noteworthy variables, such as reasons for a particular treatment, elective nodal coverage, premature cessation of therapy, and salvage treatments. Although receipt of chemotherapy is recorded, specific agents are not mentioned. Although the NCDB has a record of surgical margins, this information is very frequently missing; it also does not record other endpoints such as tolerance of therapy (including specific postoperative complications or toxicities in general), cancer-specific survival, and local/regional control. Third, the inclusion of T1-2N0 patients (similar to the CROSS study) may bias towards no differences between groups, as patients more likely to benefit from ARTs may be more advanced/bulky cases. Fourth, these results were not performed in the definitive CRT setting and do not apply to this circumstance (36,40). Nevertheless, the caveats herein do not obviate the need for further investigation to corroborate these conclusions.
Conclusions
Study of a contemporary national database illustrates that utilization of ARTs (IMRT in the vast majority) is steadily rising in the United States, and 3DCRT is now used in a minority of patients. This has implications for payers and insurance coverage going forward. ART use is impacted by not only age and disease factors, but also regional and facility differences. As expected, there was no influence of technique on OS, and also did not display apparent differences in postoperative events. Trimodality therapy at academic institutions is independently associated with higher survival, which has implications for patient counseling.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
References
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer 2016;122:1483-501. [Crossref] [PubMed]
- Verma V, Shah C, Rwigema JC, et al. Cost-comparativeness of proton versus photon therapy. Chin Clin Oncol 2016;5:56. [Crossref] [PubMed]
- Chuong MD, Hallemeier CL, Jabbour SK, et al. Improving outcomes for esophageal cancer using proton beam therapy. Int J Radiat Oncol Biol Phys 2016;95:488-97. [Crossref] [PubMed]
- Verma V, Lin SH, Simone CB 2nd, et al. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol 2016;7:644-64. [Crossref] [PubMed]
- Verma V, Moreno AC, Lin SH. Advances in radiotherapy management of esophageal cancer. J Clin Med 2016;5:E91. [Crossref] [PubMed]
- Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol 2005;77:247-53. [Crossref] [PubMed]
- Ghosh S, Kapoor R, Gupta R, et al. An evaluation of three dimensional conformal radiation therapy versus intensity modulated radiation therapy in radical chemoradiation of esophageal cancer: a dosimetric study. Clin Cancer Invest J 2012;1:65-70. [Crossref]
- Kole TP, Aghayere O, Kwah J, et al. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:1580-6. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers. Version 1.2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophgeal.pdf
- Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078-85. [Crossref] [PubMed]
- Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2013;86:885-91. [Crossref] [PubMed]
- Lin SH, Zhang N, Godby J, et al. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer 2016;122:917-28. [Crossref] [PubMed]
- Lin SH, Merrell KW, Shen J, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol 2017;123:376-81. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non–small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Gharzai L, Verma V, Denniston KA, et al. Radiation therapy and cardiac death in long-term survivors of esophageal cancer: an analysis of the surveillance, epidemiology, and end result database. PLoS One 2016;11:e0158916. [Crossref] [PubMed]
- Verma V, Mehta MP. Clinical outcomes of proton radiotherapy for uveal melanoma. Clin Oncol (R Coll Radiol) 2016;28:e17-27. [Crossref] [PubMed]
- Verma V, Shah C, Mehta MP. Clinical outcomes and toxicity of proton radiotherapy for breast cancer. Clin Breast Cancer 2016;16:145-54. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Wahl AO, et al. Proton radiotherapy for gynecologic neoplasms. Acta Oncol 2016;55:1257-65. [Crossref] [PubMed]
- Verma V, Iftekaruddin Z, Badar N, et al. Proton beam radiotherapy as part of comprehensive regional nodal irradiation for locally advanced breast cancer. Radiother Oncol 2017;123:294-8. [Crossref] [PubMed]
- Rwigema JM, Verma V, Lin L, et al. Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer 2017;123:4244-51. [Crossref] [PubMed]
- Chang JY, Verma V, Li M, et al. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non-small cell lung cancer: final results of a phase 2 study. JAMA Oncol 2017;3:e172032. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: Analysis of the National Cancer Data Base. Gynecol Oncol 2017;144:28-33. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, Teh BS. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol 2017;133:369-75. [Crossref] [PubMed]
- Moreno AC, Verma V, Hofstetter WL, et al. Patterns of care and treatment outcomes of elderly patients with stage i esophageal cancer: analysis of the national cancer data base. J Thorac Oncol 2017;12:1152-60. [Crossref] [PubMed]
- Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;97:146-54. [Crossref] [PubMed]
- McMillan MT, Ojerholm E, Verma V, et al. Radiation treatment time and overall survival in locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;98:1142-52. [Crossref] [PubMed]
- Verma V, Ryckman JM, Simone CB 2nd, et al. Patterns of care and outcomes with the addition of chemotherapy to radiation therapy for stage I nasopharyngeal cancer. Acta Oncol 2018;57:257-61. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. National cancer data base report on pneumonectomy versus lung-sparing surgery for malignant pleural mesothelioma. J Thorac Oncol 2017;12:1704-14. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- Brower JV, Chen S, Bassetti MF, et al. Radiation dose escalation in esophageal cancer revisited: a contemporary analysis of the National Cancer Data Base, 2004 to 2012. Int J Radiat Oncol Biol Phys 2016;96:985-93. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol 2017;135:173-81. [Crossref] [PubMed]
- Haefner MF, Lang K, Verma V, et al. Intensity-mdulated versus 3-dimensional conformal radiotherapy in the definitive treatment of esophageal cancer: comparison of outcomes and acute toxicity. Radiat Oncol 2017;12:131. [Crossref] [PubMed]
- Freilich J, Hoffe SE, Almhanna K, et al. Comparative outcomes for three-dimensional conformal versus intensity-modulated radiation therapy for esophageal cancer. Dis Esophagus 2015;28:352-7. [Crossref] [PubMed]
- Yang H, Feng C, Cai BN, et al. Comparison of three-dimensional conformal radiation therapy, intensity-modulated radiation therapy, and volumetric-modulated arc therapy in the treatment of cervical esophageal carcinoma. Dis Esophagus 2017;30:1-8. [PubMed]
- ClinicalTrials.gov. Proton Beam therapy (PBT) versus intensity-modulated radiation therapy (IMRT) trial. Available online: https://clinicaltrials.gov/ct2/show/NCT01512589
- Post CM, Haefner MF, Lin C, et al. Neoadjuvant versus definitive chemoradiation for locally advanced esophageal squamous cell carcinoma. Transl Cancer Res 2017;6:S625-8. [Crossref]