Cost-utility analysis of 5-fluorouracil and capecitabine for adjuvant treatment in locally advanced rectal cancer
Introduction
Colorectal cancer (CRC) is the third common cause of malignancy in the world. The incidence and death rate from this cancer has a trend increasingly, especially in developing countries (1). Chemotherapy and radiation therapy (RT) are considered as appropriated pre-operative and post-operative treatments for locally advanced rectal cancer (LARC), stage II (T3–4N0M0) and III (T1–4N1–2M0). Intravenous administration of 5-fluorouracil (5-FU)-based regimen or oral administration of fluoropyrimidine as capecitabine is commonly used in concurrent time with RT and adjuvant treatment. In 2012, a landmark study from Germany reported that capecitabine could be substituted for 5-FU in pre-operative or post-operative setting for rectal cancer because of its non-inferiority of efficacy and less hemato-toxicity than 5-FU (2). Therefore, capecitabine is proved to be used as an adjuvant setting in NCCN guideline for rectal cancer (3).
Besides the aspect of treatment efficacy, cost of treatment is another issue that must be concerned. Considering the cost of treatment, capecitabine is much more expensive than 5-FU-based regimen including administration as intravenous bolus (Mayo Clinic regimen) or 5-day continuous infusion (CI) during RT (CAO/ARO/AIO-94 protocol) (4). Since 2000, several economic analysis from western countries compared cost-effectiveness of these two drugs for colorectal cancer including palliative setting in provider viewpoint (5), third-party viewpoint (6), societal viewpoint (7) as well as adjuvant setting for colon cancer from both National Health Service viewpoint and societal viewpoint (8). All results showed that both direct medical cost and direct non-medical cost of capecitabine were lower than 5-FU in terms of hospitalization during drug administration, costs of treatment from side effects of chemotherapy, travelling costs and loss of income during treatment. The pharmacoeconomic studies of colorectal cancer from Netherland confirmed that capecitabine was the cost-saving regimen comparing with 5-FU regimens in the western countries (9,10). In addition, the studies in Asia including Taiwan and Japan also reported the similar results which capecitabine was the drug of choice because of its lower costs and better treatment outcomes than 5-FU plus leucovorin (LV) in adjuvant setting for colon cancer (11,12). For LARC, nature of the disease and treatment are not the same as locally advanced colon cancer. Nowadays, there is no evidence of the pharmacoeconomic analysis concerning the variation of adjuvant chemotherapy at the concurrent time with RT as well as at the timing of adjuvant chemotherapy alone. Moreover, the contexts of health policy, costs, and economic situation of each country are substantial difference. In Thailand, locally advanced colorectal cancer patients who used Universal Coverage Scheme and Social Security Scheme are not allowed to use capecitabine in any indication because of its price without considering other direct medical costs (cost of drug administration and management of adverse events) as well as direct non-medical costs which also affect patients. Therefore, the objective of this study was to evaluate a cost utility analysis of LARC patients who received adjuvant chemotherapy of 5-FU plus LV for 5 days per cycle (Mayo Clinic regimen), 5-FU CI for 120-hour per cycle (CAO/ARO/AIO-94 protocol) or capecitabine in the aspect of provider and societal viewpoint.
Methods
Study design and model
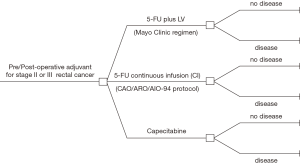
A decision tree model was conducted to compare the cost and utility of locally advanced or stage II or stage III rectal cancer patients who received pre-operative or post-operative concurrent chemoradiotherapy (CCRT) and adjuvant chemotherapy between 5-FU and capecitabine (Figure 1). Regimens of 5-FU using in this study were based on the common using and feasibility as Mayo Clinic regimen (5-FU 370–425 mg/m2 plus LV 20–25 mg/m2 intravenous bolus for 5 days) or CAO/ARO/AIO-94 protocol (1,000 mg/m2 CI in 24 hours per day for 5 days), and both regimens were given every 4 weeks for 24 weeks from concurrent time with RT therapy through adjuvant time by chemotherapy alone. For capecitabine, dose during RT was 1,650 mg/m2 per day and 2,500 mg/m2 per day after completed RT for 14 days every 21 days for a total time of 24 weeks. Follow up of all patients after completing treatment was followed by the physical examination and carcinoembryonic antigen (CEA) protocol every 3 months for the first 2 years and then every 6 months for the last 3 years. Colonoscopy and computed tomography of abdominal and pelvis was taken once a year. Costs and utility were assumed to be equal for all patients who were alive without the disease regardless of receiving either 5-FU or capecitabine and state as “no disease”. Patients with disease progression would be stated as “disease” with the hypothesis that these patients could not be obtained salvage or curative surgery. Palliative chemotherapy as FOLFOX4 regimen (5-FU plus LV and oxaliplatin), which is the most cost-effective regimen in context of Thailand (13) with the survival time of 18 months (14), was provided. The time horizon of this study was 5 years. All clinical data using in the model were conducted from literature review. Direct medical costs were the cost from database of Drug Medical Supply Information Center (15) and Heath Intervention and Technology Assessment Program (HITAP) (16), while direct non-medical cost and utility were interviewed from stage II and III rectal cancer patients. All detail will be described later. Incremental cost-effectiveness ratio (ICER) was the final result in this study, which determined as the numerator of the difference of costs among three drug regimens, and the difference of quality-adjusted life years (QALYs) from each drug was the denominator.
Parameters
Clinical parameters
The qualification of literatures, which was used for clinical outcomes in this study, was based on randomized controlled trial (RCT). Clinical outcome using for analysis in this model was 5-year disease-free survival (DFS). This data was provided from the landmark study of Hofheinz et al. (2) to determine the probability of “no disease” and “disease” at 5 years. Patients who administrated 5-FU intravenous bolus plus LV (Mayo Clinic regimen) or CI (CAO/ARO/AIO-94 protocol) were presumed to have the same survival outcomes. Therefore, the parameter for 5-year DFS was 68% (95% CI, 60–74) and 54% (95% CI, 45–62) in the capecitabine and both 5-FU regimens, respectively. For probability of grade 3–4 toxicities for each drug, data was extracted from the relevant studies and was demonstrated in Table 1.

Full table
Costs and utility
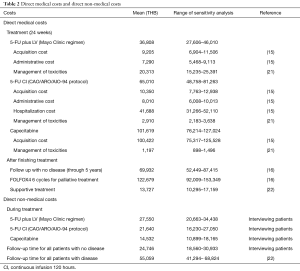
This economic study was analyzed in provider and societal viewpoints. The specific period for data collection of costs and utility was started from the first time each patient receiving chemotherapy either pre-operative or post-operative CCRT to through 5-year follow up. All occurred costs beyond the year of 2015 were computed to be the costs of that year by using discount rate of 3%, which presented in Thai Baht (THB) and US$ (the average currency exchange rate was approximately 34 THB/US $). Direct medical costs were costs of chemotherapy (with assumption of body surface area of patients =1.5 m2), chemotherapy administrative costs (5-FU plus LV bolus and 5-FU CI), costs of hospitalization (5-FU CI), costs of treatment for grade 3–4 side effects, costs of all investigations during follow-up period, costs of palliative chemotherapy (FOLFOX4 6 cycles) and symptomatic treatment. All direct medical costs were based on the database of Drug Medical Supply Information Center (15) and HITAP (16). Costs which were identical to irrespective of chemotherapy regimen were excluded including costs at diagnosis timing, costs of operation and costs of RT.
For direct non-medical cost and utility, all data were collected concurrently from stage II or III rectal cancer patients who received treatment as pre-operative/post-operative CCRT or were at follow-up period from four tertiary hospitals (Faculty of Medicine Vajira Hospital, Faculty of Medicine of Chiang Mai and Prince of Songkla University, Bhumibol Adulyadej Hospital) and three cancer hospitals (Udonthani, Lampang, Chonburi Cancer Hospital) during January 2015 to December 2015 after an approval from the Ethics Committee for Research involving Human Subjects of each institution. The data of direct non-medical costs, which were the burden costs of patients, family and caregivers, including travelling cost, food and income loss during the treatment of rectal cancer were interviewed from patients. Utility was obtained from the Euro-Quality of Life Five-Dimension-Thai version (EQ-5D-TH) questionnaire (registered at the EuroQol website), and converted to utility scores (18-20). Then the scores were multiplied by life year gained for each chemotherapy regimen to be QALYs. Data of all costs was shown in Table 2, and data of utility was demonstrated in Table 3.
Full table

Full table
Sensitivity analysis
One-way sensitivity was conducted to modify the ICER from the uncertainty of each parameter. We considered the variation of all costs between 75% and 125%, lower and upper value of 95% CI (±1.96 of standard error) for all clinical outcomes and utility, and range of 0% to 6% for discount rates (24). Probabilistic analysis was used to observe the difference of ICER when all parameters were changed at the same time.
Results
From January 2015 to December 2015, 60 stage II or III rectal cancer patients who received pre-operative or post-operative CCRT and adjuvant chemotherapy (5-FU plus LV in 13, 5-FU CI in 15, capecitabine in 32) and 85 rectal cancer patients who finished the treatment and received a surveillance were interviewed about direct non-medical costs and utility during the follow-up period. From the provider viewpoint, the costs throughout 5 years for 5-FU plus LV, 5-FU CI and capecitabine per patient were 153,452 THB (US $4,513), 181,654 THB (US $5,343) and 202,221 THB (US $5,948), respectively. Moreover, higher figures were reported from the societal viewpoint, 222,720 THB (US $6,551) for 5-FU plus LV, 245,013 THB (US $7,206) for 5-FU CI, and 253,306 THB (US $7,450) for capecitabine. The QALYs for 5 years were 3.00 for 5-FU plus LV, 3.09 for 5-FU CI and 3.26 for capecitabine. Due to the lowest cost and QALY, 5-FU plus LV was used as a comparator. From the provider viewpoint, the ICERs of 5-FU CI and capecitabine were 334,550 THB/QALY (US $9,840/QALY) and 189,935 THB/QALY (US $5,586/QALY), respectively, with the corresponding societal viewpoint of 264,447 THB/QALY (US $7,778/QALY) and 119,120 THB/QALY (US $3,504/QALY).
Sensitivity analysis
At the willingness to pay for one QALY gained in Thailand threshold of 160,000 THB/QALY or US $4,706, acquisition cost of capecitabine was the most influential parameter for value of the treatment by capecitabine when each parameter was adjusted independently in the variation as previously mention. The 25% reduction from the baseline cost of capecitabine’s acquisition cost decreased the cost to gain one QALY by approximately 51% (92,157 THB/QALY) in provider perspective and 82% (21,342 THB/QALY) in societal perspective. Considering the range of ICER, the discount cost of capecitabine in the provider viewpoint was 111,712 THB for 20%, 131,270 THB for 15%, 150,822 THB for 10% and 170,376 for 5%. Probability of 5-year DFS of each drug was also had a great impact on ICER. The first three parameters with the maximum impact on ICER were demonstrated in Table 4.

Full table
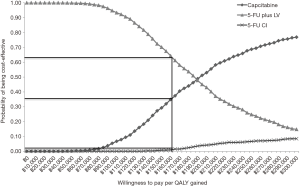
Probabilistic analysis was performed by Monte Carlo simulation 1,000 times to illustrate the probabilities of cost-effectiveness in provider perspective when all drug regimens were compared. When the willingness to pay of Thailand was 160,000 THB, 5-FU plus LV, 5-FU CI and capecitabine had probabilities of cost-effectiveness of 63%, 2% and 35%, respectively. The result was demonstrated in Figure 2.
Discussion
The results in this study showed that 5-FU plus LV was the cheapest and least effective regimen for adjuvant treatment of LARC in both provider and societal viewpoint, whereas capecitabine was the most expensive with higher efficacy than 5-FU plus LV and 5-FU CI. Moreover, administration of 5-FU as CI for 5 days per cycle in a hospital had the highest ICER to obtain one QALY. Although the QALY rose slightly when compared with 5-FU plus LV, the treatment cost was higher due to the payment from hospitalization. Therefore, 5-FU CI was the least favorable regimen for adjuvant setting in LARC. From provider’s viewpoint, capecitabine has ICER more than the Thai ceiling ratio due to its high cost, but this value was still in the range of one to 3-folds of that ratio. However, by 10% reduction of the baseline cost of acquisition cost of capecitabine, this drug was an effective treatment in adjuvant indication for rectal cancer by one-way of sensitivity analysis. Moreover, when direct non-medical cost or incurring cost of patients and families was considered, capecitabine was the drug that could reduce these expenses the most; while the highest cost was produced by 5-FU plus LV regimen. Due to the 2-fold difference of direct non-medical cost between 5-FU plus LV and capecitabine, the ICER of capecitabine was diminished to 119,120 THB/QALY in societal viewpoint, and less than the threshold of willingness to pay for obtaining one QALY in Thailand (160,000 THB). Probabilities of 5-year DFS of 5-FU regimen and capecitabine also had an impact on ICER. In this study, this parameter was used according to the landmark clinical study of Germany (2) with minor changes concerning administered of 5-FU. In our study, 5-FU CI 1,000 mg/m2/day for 5 days was provided not only at concurrent time with RT, but also at adjuvant time for chemotherapy alone, whereas 5-FU 500 mg2/day bolus for 5 days was given after the completion of CCRT in pre-operative setting in the original study. Moreover, one regimen that was addressed in the current study was Mayo Clinic regimen, which was not used in the landmark study. Therefore, the probability of 5-year DFS of two different administrations of 5-FU in our study, which was assumed to be equal to 5-FU arm in the study of Germany, is one of our limitations.
In this study, FOLFOX, which is a regimen increasingly used in adjuvant setting of LARC and is approved to use in Thailand, was not included in the analytical model. Although some previous studies showed benefits of FOLFOX over 5-FU administration as Mayo Clinic regimen (25) or CAO/ARO/AIO-04 protocol (intravenous bolus of 5-FU 500 mg/m2 per day for 5 days per cycle) (26) at adjuvant time after receiving pre-operative CCRT in terms of 3-year DFS, we cannot deny that 5-FU has a short half-life (less than 30 minutes) when using as an intravenous bolus form. Therefore, it is expected that an additional chemotherapy by oxaliplatin could enhance DFS when compared with 5-FU alone or biochemical modulation of 5-FU by LV in those two clinical trials (25,26). On the contrary, the DFS of an addition of oxaliplatin to capecitabine (CAPOX or XELOX) was comparable to that of capecitabine alone for LARC patients in PETACC6 phase-III trial (27). Furthermore, two recent studies reported the implication of oxaliplatin as adjuvant chemotherapy (FOLFOX) in these patients and concluded that using oxaliplatin as an additional drug to 5-FU should be prudent due to the inappropriate comparator and non-homogeneous among trials even though this drug may increase DFS (28,29). Nowadays there is no evidence of efficacy comparison between FOLFOX and capecitabine in the indication of adjuvant therapy, but an updated meta-analysis between FOLFOX and XELOX in metastatic colorectal cancer confirmed the equivalent of these two regimens in overall survival (OS) (30). From these data, it is possible to claim that FOLFOX and capecitabine has equal efficacy when apply these regimens in the adjuvant time. However, oxaliplatin can cause chronic sensory neuropathy, which can affect patients’ quality of life (31). Consequently, FOLFOX regimen was excluded from data analysis in our model because of its high cost and less utility.
For more than 15 years, many economic studies have proved that capecitabine is the cost-saving drug over Mayo Clinic regimen in colon cancer (6-8,11,12). Although the substitution of capecitabine for 5-FU was confirmed to use in adjuvant setting for rectal cancer, the economic evaluation is not taken place to confirm the value of money in this situation. This study was the first study to evaluate the value of capecitabine as the indication of adjuvant therapy in rectal cancer, thereby there were some different aspects worth mentioning between our study and previous studies focused on colon cancer as followed. The treatment outcomes of locally advanced stages of colon cancer and rectal cancer patients who received adjuvant therapy were not equal, and had effects on the probability of survival outcomes in the model (2,32). In addition, the difference of surgical technique (half colectomy vs. low anterior resection vs. abdominoperineal resection vs. total mesorectal excision) between colon cancer and rectal cancer were observed. Moreover, RT is not a conventional treatment for adjuvant setting of colon cancer, but this treatment modality has indication to use in LARC in pre-operative of post-operative timing. Therefore, the data of quality of life of colon cancer patients, which is influenced by consequences of different treatments as well as side effects during the treatment, cannot be used for rectal cancer patients and vice versa.
Our results reported not only similar but also different findings from the preceding studies. Generally, an acquisition cost of capecitabine was higher than 5-FU based regimen irrespective of type of cancer. When administration costs which was consisted of the direct medical cost was considered, however, the different finding was emerged. Since the administration costs as well as hospitalization cost in Thailand were much lower than the western countries, inclusion these costs in direct medical cost had no effect on the total direct medical cost; while high cost of administration of 5-FU for Mayo Clinic regimen could bring all direct medical cost to be higher than capecitabine in both western countries and Taiwan (8,11).
There are other limitations in this study besides the probability of 5-year DFS of 5-FU regimen. The probabilities of grade 3–4 toxicities of Mayo Clinic regimen were obtained from another study (17), whereas toxicity data of 5-FU CI and capecitabine were available from the study of Hofheinz et al. (2). Therefore, these probabilities were indirectly compared between two studies and might not be appropriated, and could be factors influencing the direct medical cost of Mayo Clinic regimen, which the manageable cost of toxicities was about 55%. Additionally, there were small numbers of patients who were interviewed during treatment in both 5-FU regimens. It was probably inadequate to be the real representation of direct non-medical costs and quality of life. However, this period lasted only 5–6 weeks, so it might have minimal effect on the entire costs and utility.
There are variations of government policy, social welfare, living expenses and access to medical care in each country. Therefore, the analysis of effectiveness for one treatment of the same disease from one country cannot apply to other countries. The ceiling value of willingness to pay per QALY is one of the most important figures contributed to the decision making for the efficacy of treatment, and this value is wildly different among countries. With the acceptability threshold as 160,000 THB (US $4,706) for Thailand, the cost of capecitabine was slightly higher than the budget in provider’s viewpoint. However, the cost was acceptable when the acquisition cost of capecitabine was reduced by 10%. From societal viewpoint, on the other hand, capecitabine was the drug that reduced the burden of patients and families from other costs which was occurred during treatment. In real situation, some patients are lost from treatment due to the financial problem. The decision making of treatment of choices should not focus only on cost saving for the provider, but also the affordability of patients and their family. In developing countries including Thailand, the limitation of resources and the equality for all patients to receive the standard treatment are difficult to keep them in balance. Consideration of the effects on all perspectives before determination of health care policy is needed deliberately for policy makers to confirm the fairness of all cancer patients.
Conclusions
5-FU plus LV was the cheapest and least efficacy for adjuvant treatment of LARC in both provider and societal viewpoint, whereas capecitabine was the most expensive with higher effectiveness than 5-FU plus LV and 5-FU CI. Importantly, using capecitabine can reduce direct non-medical costs, which are burden for patients and families especially in Thailand. Thus, this issue should be considered for policy makers before making decision.
Acknowledgements
This work was supported by medical research fund of Faculty of Medicine Vajira Hospital, Navamindradhiraj University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee for Research involving Human Subjects of Faculty of Medicine Vajira Hospital, with protocol number of COA 80/2557. All patients have been obtained the inform consent before stating treatment or interview.
References
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683-91. [Crossref] [PubMed]
- Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. [Crossref] [PubMed]
- NCCN Clinical Practice Guideline for rectal cancer Version 2.2016. Accessed 10/8/2017. Available online: https://www.tri-kobe.org/nccn/guideline/colorectal/english/rectal.pdf
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Twelves C, Boyer M, Findlay M, et al. Capecitabine (XelodaTM) improves medical resource use compared with 5-fluorouracil plus leucovorin in a phase III trial conducted in patients with advanced colorectal carcinoma. Eur J Cancer 2001;37:597-604. [Crossref] [PubMed]
- Hieke K, Kleeberg UR, Stauch M, et al. Costs of treatment of colorectal cancer in different settings in Germany. Eur J Health Econ 2004;5:270-3. [Crossref] [PubMed]
- Jansman FG, Postma MJ, van Hartskamp D, et al. Cost-benefit analysis of capecitabine versus 5-fluorouracil/leucovorin in the treatment of colorectal cancer in the Netherlands. Clin Ther 2004;26:579-89. [Crossref] [PubMed]
- Cassidy J, Douillard JY, Twelves C, et al. Pharmacoeconomic analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes’ C colon cancer: the X-ACT trial. Br J Cancer 2006;94:1122-9. [Crossref] [PubMed]
- Krol M, Koopman M, Uyl-de Groot C, et al. A systematic review of economic analyses of pharmaceutical therapies for advanced colorectal cancer. Expert Opin Pharmacother 2007;8:1313-28. [Crossref] [PubMed]
- Jansman FG, Postma MJ, Brouwers JR. Cost considerations in the treatment of colorectal cancer. Pharmacoeconomics 2007;25:537-62. [Crossref] [PubMed]
- Hsu TC, Chen HH, Yang MC, et al. Pharmacoeconomic analysis of capecitabine versus 5-fluorouracil/leucovorin as adjuvant therapy for stage III colon cancer in Taiwan. Value Health 2011;14:647-51. [Crossref] [PubMed]
- Shiroiwa T, Fukuda T, Shimozuma K, et al. Cost-effectiveness analysis of capecitabine compared with bolus 5-fluorouracil/l-leucovarin for the adjuvant treatment of colon cancer in Japan. Pharmacoeconomics 2009;27:597-608. [Crossref] [PubMed]
- Economic evaluation of adjuvant chemotherapy in patients with resectable metastatic colorectal cancer in Thailand. Accessed 10/8/2017. Available online: http://www.hitap.net/documents/163949
- Giessen C, Laubender RP, Ankerst DP, et al. Progression-free survival as a surrogate endpoint for median overall survival in metastatic colorectal cancer: Literature-based analysis from 50 randomized first-line trials. Clin Cancer Res 2013;19:225-35. [Crossref] [PubMed]
- Drug and Medical Supply Information Center, Ministry of Public Health. Accessed 10/8/2017. Available online: http://dmsic.moph.go.th/dmsic/index.php?p=1&id=22&b_id=49&sec=2
- Heath Intervention and Technology Assessment Program. Accessed 10/8/2017. Available online: http://costingmenu.hitap.net/
- Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol 2005;16:549-57. [Crossref] [PubMed]
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. [Crossref] [PubMed]
- EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. [Crossref] [PubMed]
- Tongsiri S, Cairns J. Estimating population-based values for EQ- 5D health states in Thailand. Value Health 2011;14:1142-5. [Crossref] [PubMed]
- Riewpaiboon A. Standard cost lists for health technology assessment. Bangkok: HITAP 2011.
- Lerdkiattikorn P, Chaikledkaew U, Lausoontornsiri W, et al. Cost-utility analysis of adjuvant chemotherapy in patients with stage III colon cancer in Thailand. Expert Rev Pharmacoecon Outcomes Res 2015;15:687-700. [Crossref] [PubMed]
- Ness RM, Holmes AM, Klein R, et al. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol 1999;94:1650-7. [Crossref] [PubMed]
- Chaikledkaew U, Teerawattananon Y, Suksomboon N. Health intervention and technology assessment program. Nonthaburi, Thailand: Graphico Systems Co.; 2009.
- Hong YS, Nam B-H, Kim K-P, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 2014;15:1245-53. [Crossref] [PubMed]
- Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979-89. [Crossref] [PubMed]
- Schmoll HJ, Haustermans K, Price T, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin vs. capecitabine alone in locally advancedrectal cancer: disease free survival results at interim analysis. Eur J Cancer 2014;50:S1-8. [Crossref]
- Zhao L, Liu R, Zhang Z, et al. Oxaliplatin/fluorouracil-based adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery: a systematic review and meta-analysis of randomized controlled trials. Colorectal Dis 2016;18:763-72. [Crossref] [PubMed]
- Netter J, Douard R, Durdux C, et al. Advances in management of adjuvant chemotherapy in rectal cancer: Consequences for clinical practice. Clin Res Hepatol Gastroenterol 2016;40:546-52. [Crossref] [PubMed]
- Guo Y, Xiong BH, Zhang T, et al. XELOX vs. FOLFOX in metastatic colorectal cancer: An updated meta-analysis. Cancer Invest 2016;34:94-104. [Crossref] [PubMed]
- Stefansson M, Nygren P. Oxaliplatin added to fluoropyrimidine for adjuvant treatment of colorectal cancer is associated with long-term impairment of peripheral nerve sensory function and quality of life. Acta Oncol 2016;55:1227-35. [Crossref] [PubMed]
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as Adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. [Crossref] [PubMed]