Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in ovarian and gastrointestinal peritoneal carcinomatosis: results from a 7-year experience
Introduction
Peritoneal carcinosis (PC) remains a poor prognosis disease. However several steps have been made to understand PC and to prevent it.
Many studies reported worst prognosis especially for secondary PC from some tumors like gastric cancer (GC). Others tumors as colon and appendiceal cancer (AC) seem to have better outcomes especially if the diagnosis is performed precociously. PC from pseudomyxoma peritonei [or disseminated peritoneal adenomucinosis (DPAM)] has a very good response to the local treatment with survival rates at 10 years more than 63% (1). On the other hand, others PC from less common diseases are currently being studied to evaluate the role of local treatments [cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC)]. Gynecological tumors, especially ovarian cancer (OC), have been studied for first and for a longer time. However until today there were no definitive data. A recent randomized controlled trial (RCT) published in N Engl J Med (2) about 245 patients with advanced-stage OC reported that the addition of HIPEC to interval cytoreductive surgery resulted in longer recurrence-free survival and overall survival (OS) than surgery alone and did not result in higher rates of side effects.
However, despite the histological differences between various tumors, very good results are reported in literature in the treatment of PC compared to no-treat approach.
The key point for the success and the good outcomes is still reaching the completeness of cytoreduction (CC) removing all macroscopic disease and treating the microscopic tumor cells in peritoneal cavity with the intraperitoneal chemotherapy, both with a therapeutic or a prophylactic intent.
The main prognostic factors, according to the literature, are the peritoneal cancer index (PCI) and the CC.
In this paper our 7 years’ activity in treatment of PC in Papa Giovanni XXIII Hospital in Bergamo is reported. Morbidity, mortality and re-operations rates of about 150 cases of PC from different origins (gastrointestinal, gynecological and others less common tumors) are analyzed.
Methods
The study included a total of 150 patients treated with CRS combined with HIPEC from January 2011 to July 2017 in Papa Giovanni XXIII Hospital. This is a prospective observational study focusing on patients undergoing HIPEC for different gastrointestinal tumors {colorectal cancer (CRC), AC [divided into DPAM and peritoneal mucinous carcinomatosis (PMCA)], high-grade non-mucinous carcinoma (PCA), GC}, gynecological tumors (OC and uterine cancer) and other tumors (sarcomas, duodenal tumor, histiocytoma, breast tumor, mesothelioma, colangiocarcinoma).
All patients had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1, without extra-abdominal disease. According with the pre-operative images [computed tomography, positron emission tomography (PET), magnetic resonance, endoscopic ultrasound, oesophagogastric endoscopy, colonoscopy, exploratory laparoscopy] the peritoneal disease was debulkable. Patients with hepatic metastases were included only if hepatic lesions were easily resectable (with wedge or segmentectomy resections) and they were treated with the collaboration of expert hepatobiliary surgeons.
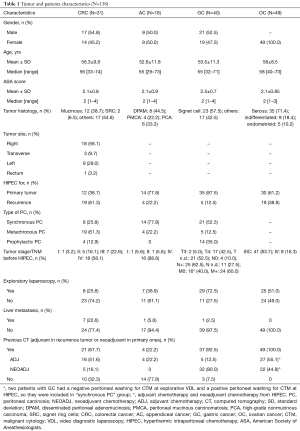
Patients, tumors characteristics and pre-operative data are reported in Table 1 for the different pathologies. Collected data were: age, gender, tumor histology, American Society of Anesthesiologists (ASA) score, primary tumor or recurrence, synchronous or metachronous PC, tumor stage (TNM) before HIPEC, presence or absence of liver metastasis, neoadjuvant chemotherapy in primary tumors or adjuvant chemotherapy for recurrent tumors, exploratory laparoscopy.
Full table
The specimens from AC were classified according to the 2010 World Health Organization classification and the 7th American Joint Committee on Cancer (AJCC) (3) in DPAM, PMCA and PCA. The first one is a peritoneal lesion characterized by large extracellular mucin with rare atypia originated from an appendiceal mucinous adenoma. PMCA is defined when the primary tumor is an appendiceal adenocarcinoma with peritoneal lesions associated with a cytology atypia. This tumor is considered more aggressive than DPAM for the potentiality to give lymphatic, hematic and peritoneal metastases (4). PCA is a high-grade non-mucinous neoplasm associated with severe cytologic atypia and mitoses (5).
CRC, GC and OC were classified according to the 7th TNM classification and the International Federation of Gynecology and Obstetrics (FIGO) classification respectively (6-8).
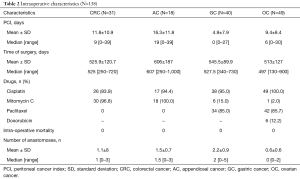
Intra-operative data are collected in Table 2: PCI at the CRS (according to Sugarbacker’s classification), operative time, drugs used, intra-operative mortality and number of intestinal resections. CRS was performed removing all peritoneum and visceral organs involved by the tumor. The omentectomy and cholecystectomy were routinely performed. The CC was graded by the surgeon at the conclusion of the procedure according to the Sugarbacker’s classification: CC0—complete cytoreduction of all visible disease; CC1—minimal residual disease with nodules less than 2.5 cm; CC2—residual disease with nodules of 2.5 mm to 2.5 cm; and CC3—residual disease with nodules greater than 2.5 cm. In all patients a CC0–1 resection was achieve, except for one patient with OC (CC2).
Full table
HIPEC technique used was the coliseum technique performed in most cases for 90 minutes with an inflow temperature of 42–43 °C and an outflow temperature of 40–41 °C. One inflow and four outflow catheters were placed with the open abdomen that was partially closed with a surgical adhesive drape performing a “closed-HIPEC with open abdomen technique”. Chemotherapy regimens were: 35 mg/m2 for mitomycin-C (MMC) (or 16 mg/m2 MMC if cisplatin was added), 100 mg/m2 for cisplatinum and 175 mg/m2 for paclitaxel, adriblastin 15 mg/L of perfusate. Afterward, the perfusate is drained and the reconstructive time was performed.
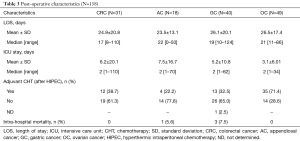
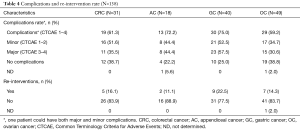
Post-operative data and outcomes were collected in Tables 3-5 and in Figures 1-4. Data taking into account were: intensive care unit (ICU) and hospital stay, perioperative mortality (during hospitalization), reintervention rate, adverse events [which were classified with the Common Terminology Criteria for Adverse Events (CTCAE) (9)], adjuvant chemotherapy, disease free survival (DFS), and OS. Clinical and oncological outcomes were compared between the different groups. Factors that could affect both oncological and clinical outcomes (PCI, primary site of disease, age, and ASA score) were analyzed.
Full table
Full table

Full table

Statistical analysis
Continuous and categorical variables including frequencies and percentages for categorical data were reported in Tables 1-4,6. DFS and OS were calculated as the interval between the date of CRS and HIPEC and the data of the last follow-up or of the death or of the recurrence of disease. The curves for DFS and OS were performed using the Kaplan-Meier method, and survival estimates were compared using the log-rank test. Cox-regression was used for multivariate analysis. Univariate analyzes on clinical outcomes were performed with t-test for continuous quantitative variables with normal distribution and with the Mann-Whitney test for non-normal distribution variables. Categorical variables were compared with chi-square test. Statistical significance was defined as a P value <0.005. All analysis was performed using SPSS 20 (IBM Corp., Released 2011, IBM SPSS Statistics for Windows, Version 20.0, Armonk, NY, USA).
Full table
Results
Pre-operative characteristics
In the study groups GC, CRC, AC and OC patients are equally distributed for sex as reported in Table 1, except for ovarian patients that are obviously all female. Patients had median age between 55 to 58 years.
The histology of the primary tumor is different for the different diseases as reported in Table 1.
While for appendiceal, gastric and OC patients were treated mainly for a primary tumor, in the CRC group the recurrence was more often treated (Table 1).
The exploratory laparoscopy is significantly more often performed for GC and in the half of patients with OC patients, however it is not so frequent in CRC (especially in recurrent tumors) and in ACs (Table 1).
The 22.6% of patients (n=7) with colorectal cancer had also a resectable liver metastasis and these patients were contemporary treated for both tumors with CRS and HIPEC. Liver metastases were less frequent in the other tumors.
The 80% of patients (n=32) with GC and the 44.8% of patients (n=22) with OC underwent neoadjuvant chemotherapy before HIPEC. No patient with AC underwent neoadjuvant chemotherapy instead. In CRC group only 16.1% of patients received neoadjuvant chemotherapy (NACT) before HIPEC.
Intra-operative characteristics
PCI was lower in GC [mean ± standard deviation (SD) 4.9±7.9] compared to CRC and AC (mean ± SD 11.8±10.9 and 16.3±11.8 respectively). In OC group the mean ± SD of PCI was 9.4±8.4 (P<0.001).
Time of surgery is similar in the four groups as reported in Table 2. Intraoperative mortality is 0% in all the four groups of study.
Patients in the GC group underwent a higher number of gastrointestinal anastomosis after HIPEC compared to others groups (P<0.001) as shown in Table 2.
Others cancer group characteristics
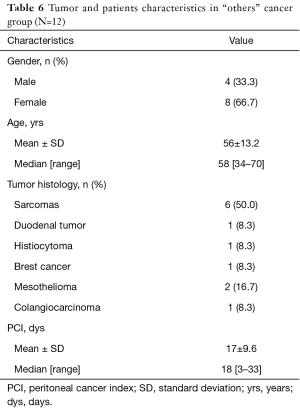
As reported in Table 6 this group includes 12 patients (4 male and 8 female). The median age was 56 years (range 34–70 years). The different pathologies treated were: 6 patients with sarcomas (1 myxoid liposarcoma, 1 leiomyosarcoma, 2 ovarian carcinosarcomas and 2 uterine carcinosarcomas), 1 duodenal tumor, 1 histiocytoma, 1 breast tumor, 2 mesotheliomas, and 1 colangiocarcinoma.
In six patients with sarcoma doxorubicin and cisplatin (in one patient was used doxorubicin alone) were used, as also in the patient with malignant histiocytoma. In the patient with duodenal tumor cisplatin and mitomycin were used. In the patient with breast cancer cisplatin and taxol; and in two patients with mesothelioma, taxol, doxorubicin, cisplatin and doxorubicin were used.
The median PCI was 18 (range between 3 and 33). No patients died during surgery.
Clinical outcomes
The length of stay wasn’t associated to the different sites of primary disease (Table 3).
Major morbidity rate was 38% (57 pts) (CTCAE 3–4). Re-operation rate was 15.3% (23 pts) and perioperative mortality (during hospitalization) was 2.7% (4 pts) (Table 3). PCI, age, ASA score or sites of primary tumor weren’t significantly associated with clinical outcomes in terms of global and minor complications rate, perioperative mortality and re-operation rate. Patients that had higher major morbidity rate were significantly younger than patients that hadn’t {mean ± SD: 52.9±11, median 54 [29–74] vs. mean ± SD: 56.6±8.9, median 58 [36–73]}. PCI, site of primary tumor and ASA score didn’t influence major complications rate.
Globally patients in OC group underwent more frequently (P<0.001) adjuvant chemotherapy (71%) than GC, AC and CRC as shown in Table 3.
Oncological outcomes
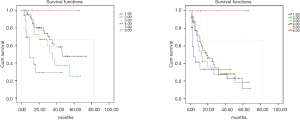
We analyzed DFS and OS in the different groups of disease as reported in Table 5.
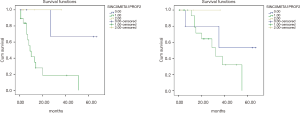
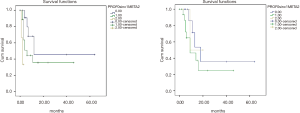
In GC group there was no significant difference in OS between synchronous peritoneal (SP), metachronous peritoneal carcinosis (MPC) and prophylactic HIPEC [3-year OS 22%, 2-year OS 50%, 5-year OS 36%, P=NS (not specified)] (Figure 3). In CRC group, comparing MPC vs. synchronous peritoneal carcinosis (SPC) vs. prophylactic HIPEC no significant differences were found either in terms of OS (5-year OS 0%, 53%, and 3-year OS 100% respectively, P=NS) (Figure 1).
In GC group there was a significant difference in DFS between SPC vs. MPC groups (5-year DFS 36% vs. 3-year OS 0%, P=0.016), but not between prophylactic HIPEC and therapeutic HIPEC (5-year DFS 46%, 3-year DFS 32%, P=NS) (Figure 3). In CRC group, comparing MPC vs. SPC groups there was a significant difference in DFS too (5-year DFS 0% and 66% respectively, P=0.02) as also comparing prophylactic HIPEC vs. SPC vs. MPC from CRC (5-year DFS 66%, 0%, 3-year DFS 100% respectively, P=0.01) (Figure 1).
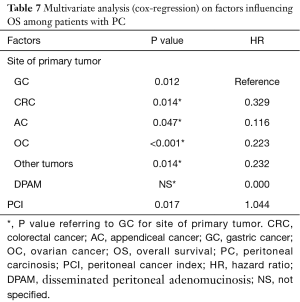
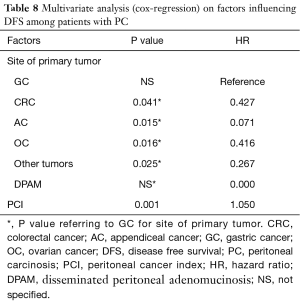
Focusing on patients with PC (patients with CRC and GC undergone prophylactic HIPEC were excluded in this phase), factors that significantly influenced oncological outcomes at multivariate analysis were the site of primary tumor (P=0.012) and the PCI value (P=0.017) for OS [PCI hazard ratio (HR) for OS adjusted for site for primary tumor: 1.044] and the PCI alone for DFS (PCI HR for DFS adjusted for site for primary tumor: 1.050, P=0.001) (Tables 7,8). Age and ASA score wasn’t significantly associated with oncological outcomes at univariate analysis.
Full table
Full table
Discussion
In the recent years we witnessed an increase interest in CRS and HIPEC to treat PC (10). The knowledge of physiopathology mechanisms of peritoneal spread, the pharmacokinetic studies, the improvement in perioperative chemotherapeutic treatments and the advancement of surgical techniques, favored the outcomes of oncological patients. These results, as reported in literature, are achieved especially in high volume centers (11,12). In fact in center with more than 300 patients the CC reaches the 80% with a low rate of laparotomy and biopsy only (1.3%) (12). Some authors reported that an experienced center should have performed at least 130 procedures (1).
However, CRS + HIPEC procedure remains a hard procedure for patients and 30-day mortality and morbidity reported in literature are 1–10% and 20–50% respectively (1).
Present study reported (about 150 CRS + HIPEC performed in our center) an overall major morbidity rate of 38% (CTCAE 3–4) and an overall perioperative mortality of 2.7% that consistent with literature (1). Clearly these results are referred to different pathologies with different tumor biology and different approach at the cytoreduction.
In the last years an increase interest for PC from GC has been motivated by the frequent peritoneal spread and by the poor prognosis of this disease. The results of present study underline the importance to prevent PC in GC to achieve better outcomes as reported also in recent studies and meta-analysis (13-17).
CRS + HIPEC in GC remains a very hard surgery for risk of malnutrition this reason it is important to perform routinely staging laparoscopy to perform a peritoneal washing, extension of surgery (almost three anastomoses should be done), high rate of patients undergone NACT. Despite the younger age of patients (median of 53 years) and the low value of PCI (mean PCI of 5) mortality and morbidity rates are still clinically higher (7.5% and 57% respectively) compared with the others diseases, even if this difference isn’t statistically different.
In colon and AC CRS + HIPEC technique is better standardized with very good long-term results [HIPEC in CRC can achieve until 20–51% 5-year OS (19–62 months of survival) and 86% 5-year OS for DPAM and 50% for PMCA (18-21)]. Patients with AC arrive to surgery with a high PCI (especially for patients with DPAM) and despite the very good outcomes results in our study [PMCA and PCA 82 months (5-year OS 67%); DPAM 5-year OS 100%], time of surgery, ICU stay, and major complication rates are higher. Also in high volume centers the morbidity due to the aggressive treatment of pseudomyxoma peritonei is reported between 22–56% with mortality near to 1–14% (21,22). In CRC group (35 months and 5-year OS 27%) most of patients had a metachronous PC. However as confirmed also by the literature the treatment of a synchronous PC are associated with better outcomes (23). No definitive results are published about the prophylactic HIPEC in CRC [COLOPEC trial is ongoing (24)]. However, looking at present data, patients with CRC at high risk for peritoneal spread (colic obstruction, perforation tumor, ovarian metastasis, positive cytology, T4 tumors, and mucinous tumors) seem to benefit significantly from CRS + HIPEC (25). Moreover as reported in a recent review it is important to take into account that 28–59% of patients with CRC can recur into peritoneum (20). Furthermore recently Sugarbaker et al. underline the importance to treat previous PC from hepatic lesions because the increase in survival is worst in patients with PC that is much less controllable than hepatic metastasis (26).
CRS + HIPEC in OC have been applied for many years to treat PC that occur in almost 70% of women (27). Moreover in about 50–75% of patients a persistent or recurrent disease can occur. However until today there were no definitive data and no clear timing to perform this treatment is define (28). The literature reports 35.4 months of survival for first-line treatment and 45.7 months for recurrent OC that are not significantly different comparing the results of CRS alone in OC patients (41.5 and 47.2 months respectively) (27). A recent RCT published in N Engl J Med (2) about 245 patients with advanced-stage OC reported that the addition of HIPEC to interval cytoreductive surgery resulted in longer recurrence-free survival and OS than surgery alone and did not result in higher rates of side effects (25% in CRS alone and 27% in CRS + HIPEC group, P=0.76). In this recent paper the median OS was 33.9 months in the surgery group compared with 45.7 months in the surgery-plus-HIPEC group, with a median recurrence-free survival of 10.7 and 14.2 months, respectively. Present data are consistent with these results (47 months and 5-year OS 48%). Another interesting data is that almost all patients with OC were treated with perioperative chemotherapy, contrary to what is happening in the others diseases. The explication is probably because gynecologists handle chemotherapy in OC patients and not oncologists that remains skeptics from HIPEC. Moreover complications rate and re-interventions rate is lower comparing with the other tumors, but not significantly.
Few studies are performed for patients underwent HIPEC for unusual primary cancer with PC (29,30). The Peritoneal Surface Oncology Group International (PSOGI) group reports a 38.5% of 5-year OS suggesting that CRS + HIPEC can be safe also in rare indications, in much selected patients (30). Others reported 30–33% of OS (29,30). These data are comparable with present data. Also in these cases CC0 and PCI are strictly related with outcomes (PCI <12 had better outcomes) (29,30). Furthermore perioperative chemotherapy is necessary to better select these patients (30). The authors found also that rare ovarian carcinoma achieve the better response to CRS + HIPEC (5-year OS 57.7% and 38.9% of DFS) that might suggest similar etiologies to the pseudomyxoma peritonei. Others studies reported that in peritoneal sarcomatosis, that could develop as recurrence in sarcoma, the combined approach with CRS (to remove macroscopic recurrent disease) and HIPEC (especially with doxorubicin and/or cisplatin, to reduce risk of another recurrence) can achieve 5-year OS of 75% (31).
In literature very few studies analyzed the impact of the site of primary tumors together with others patients characteristics (such as age, PCI and ASA score) on clinical and oncologic outcomes. Present data show that neither patients nor tumor characteristics had a real influence on clinical outcomes. However, it seems that younger patients, probably because treated with a more aggressive surgery, had higher major morbidity rate. Focusing on oncological outcomes it seems that OS is significantly influenced by both PCI and the site of primary disease, while DFS is influenced only by PCI. This can be explained by the fact that probably, while DFS strictly depends on the effect of CRS + HIPEC, the efficacy of which is correlated with PCI, OS is influenced also by the chemotherapy following the recurrence, and its efficacy is strictly related to the chemotherapy regimen and to the tumor chemosensitivity.
Despite these promising results, the study presents some bias. At first is a monocentric prospective database that evaluates outcomes of different diseases. Moreover the study covered 7 years and the increase in the number of cases and improving in the learning curve allowed getting better results especially in recent years.
Conclusions
A therapeutic approach that combined CRS + HIPEC could achieve long-term survival in selected groups of patients with PC from gastrointestinal, gynecological and others tumors with acceptable morbidity and mortality. A good expertise and a high volume of patients are necessary to manage PC and to further improve results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Bergamo’s Ethics Committee (protocol approval number Ch1BG.01) and informed consent was taken from all the patients.
References
- Wang TY, Chen CY, Lu CH, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal malignancy: preliminary results of a multi-disciplinary teamwork model in Asia. Int J Hyperthermia 2018;34:328-35. [Crossref] [PubMed]
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med 2018;378:230-40. [Crossref] [PubMed]
- Panarelli NC, Yantiss KR. Mucinous Neoplasms of the Appendix and Peritoneum. Arch Pathol Lab Med 2011;135:1261-8. [Crossref] [PubMed]
- Shankar S, Ledakis P, El Halabi H, et al. Neoplasms of the appendix: current treatment guidelines. Hematol Oncol Clin North Am 2012;26:1261-90. [Crossref] [PubMed]
- Bruin SC, Verwaal VJ, Vincent A, et al. A clinicopathologic analysis of peritoneal metastases of colorectal and appendiceal origin. Ann Surg Oncol 2010;17:2330-40. [Crossref] [PubMed]
- American Joint Committee on Cancer. Colon and Rectum Cancer Staging. Available online: https://cancerstaging.org/references-tools/quickreferences/Documents/ColonMedium.pdf
- Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:3077-9.
- World Health Organization. Classification TNM/FIGO. Available online: http://screening.iarc.fr/atlasclassiftnm.php?lang=1
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). 2009. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Coccolini F, Gheza F, Lotti M, et al. Peritoneal Carcinomatosis. World J Gastroenterol 2013;19:6979-94. [Crossref] [PubMed]
- Mohamed F, Moran BJ. Morbidity and Mortality With Cytoreductive Surgery and Intraperitoneal Chemotherapy: The Importance of a Learning Curve. Cancer J 2009;15:196-9. [Crossref] [PubMed]
- Moran B, Cecil T, Chandrakumaran K, et al. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Colorectal Dis 2015;17:772-8. [Crossref] [PubMed]
- Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12-26. [Crossref] [PubMed]
- Coccolini F, Catena F, Glehen O, et al. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur J Surg Oncol 2015;41:911-9. [Crossref] [PubMed]
- Coccolini F, Catena F, Glehen O, et al. Effect of intraperitoneal chemotherapy and peritoneal lavage in positive peritoneal cytology in gastric cancer. Systematic review and meta-analysis. Eur J Surg Oncol 2016;42:1261-7. [Crossref] [PubMed]
- Coccolini F, Celotti A, Ceresoli M, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) and neoadjuvant chemotherapy as prophylaxis of peritoneal carcinosis from advanced gastric cancer—effects on overall and disease free survival. J Gastrointest Oncol 2016;7:523-9. [Crossref] [PubMed]
- Fugazzola P, Coccolini F, Montori G, et al. Overall and disease-free survival in patients treated with CRS + HIPEC with cisplatin and paclitaxel for gastric cancer with peritoneal carcinomatosis. J Gastrointest Oncol 2017;8:572-82. [Crossref] [PubMed]
- Mirnezami R, Mehta AM, Chandrakumaran K, et al. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer 2014;111:1500-8. [Crossref] [PubMed]
- de Cuba EM, Kwakman R, Knol DL, et al. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: Systematic review of all literature and meta-analysis of observational studies. Cancer Treat Rev 2013;39:321-7. [Crossref] [PubMed]
- Mirnezami R, Moran BJ, Harvey K, et al. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal metastases. World J Gastroenterol 2014;20:14018-32. [Crossref] [PubMed]
- Shaib WL, Assi R, Shamseddine A, et al. Appendiceal Mucinous Neoplasms: Diagnosis and Management. Oncologist 2017;22:1107-16. [Crossref] [PubMed]
- Loungnarath R, Causeret S, Brigand C, et al. Pseudomyxoma peritonei: new concepts and new therapeutic approach. Ann Chir 2005;130:63-9. [Crossref] [PubMed]
- Waite K, Youssef H. The Role of Neoadjuvant and Adjuvant Systemic Chemotherapy with Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Ann Surg Oncol 2017;24:705-20. [Crossref] [PubMed]
- Klaver CE, Musters GD, Bemelman WA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer 2015;15:428. [Crossref] [PubMed]
- Honoré C, Goéré D, Souadka A, et al. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol 2013;20:183-92. [Crossref] [PubMed]
- Sugarbaker PH. Colorectal cancer: prevention and management of metastatic disease. Biomed Res Int 2014;2014:782890. [PubMed]
- Polom K, Roviello G, Generali D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for treatment of ovarian cancer. Int J Hyperthermia 2016;32:298-310. [Crossref] [PubMed]
- Ansaloni L, De Iaco P, Frigerio L. Re: "cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: multi-institutional phase II trial." - Proposal of a clinical trial of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in advanced ovarian cancer, the CHORINE study. Gynecol Oncol 2012;125:279-81. [Crossref] [PubMed]
- Honoré C, Goéré D, Macovei R, et al. Peritoneal carcinomatosis from unusual cancer origins: Is there a role for hyperthermic intraperitoneal chemotherapy? J Visc Surg 2016;153:101-7. [Crossref] [PubMed]
- Goéré D, Passot G, Gelli M, et al. Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: results from a worldwide analysis issue of the Peritoneal Surface Oncology Group International (PSOGI). Int J Hyperthermia 2017;33:520-7. [Crossref] [PubMed]
- Sugarbaker PH. Review of a personal experience in the management of carcinomatosis and sarcomatosis. Jpn J Clin Oncol 2001;31:573-83. [PubMed]


