Palliative radiation therapy for primary gastric melanoma
Introduction
Malignant melanoma is known to metastasize to visceral organs with the gastrointestinal tract (GI tract) being one of the more common sites (1). Less common, however, is primary malignant melanoma of the GI tract. Gastric melanoma can often present with vague symptoms; however, a more alarming presentation is that of an upper gastrointestinal bleeding (GI bleeding) (2,3). Case reports of primary gastric melanoma, presenting with upper GI bleeding or otherwise, have demonstrated surgery as the primary method of treatment (2-9), while the utility of radiation in the management of primary gastric melanoma has been unreported. Radiation therapy has been known to palliate bleeding of cancers of the lung, bladder and cervix (10-13), and more recently palliation of bleeding to primary gastric adenocarcinoma has been studied (14-17). The role of radiation therapy in patients with bleeding secondary to primary gastric melanoma has not yet been defined. We report a case of gastric melanoma with no identifiable cutaneous primary, treated with palliative radiation therapy for control of bleeding.
Case presentation
An 87-year-old Hispanic male presented at an outside institution with a one month history of fatigue, 10-pound weight loss, and melena. He was found to have severe anemia (Hgb 6.7) requiring transfusion. Initial CT of the abdomen and pelvis showed a possible gastric mass. Esophagogastroduodenoscopy (EGD) was performed revealing an 8 cm pedunculated mass at the greater curvature of the stomach, partly black, partly green, partly white. Endoscopic ultrasound showed an isohypoechoic heterogenous mass with visible stalk. Biopsies were taken and showed extensive, ulcerated, poorly-differentiated spindle and epithelioid cell tumor with immunohistochemistry positive for S100 and Melan-A, negative for CD117, AE1/AE3, CDX2, and BRAF mutation negative. The diagnosis of gastric malignant melanoma was made and the patient was scheduled to be seen by a surgical oncologist.
Two days after discharge from the outside facility, he presented to our institution with worsening fatigue and melena, his hemoglobin on presentation was 7.8. His bleeding was controlled and he underwent at PET/CT scan, dermatologic physical exam and ophthalmologic exam to evaluate for a primary melanoma. Dermatologic and ophthalmologic exam did not reveal a primary, PET/CT was only positive for a gastric mass with an SUV of 17. He was diagnosed with T4N0M0 Stage IIB primary gastric melanoma.
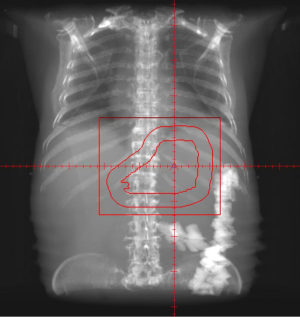
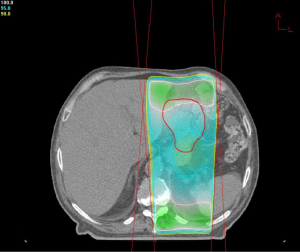
Due to the patient’s age and functional status, he was deemed unresectable and was offered palliative radiotherapy to control bleeding and anemia. He received a dose of 16 Gy to the stomach in four fractions. Following this treatment he remained hemodynamically stable for four months; at that time he presented to the emergency department with worsening fatigue, complete blood count revealed a hemoglobin of 7.0 and patient underwent further transfusion. He was offered a second course of palliative radiotherapy during which he received an additional 9 Gy to the stomach in three fractions (Figures 1,2). At the time of this writing he has tolerated his second course of therapy without complication.
Discussion
This case documents upper GI bleeding as a clinical presentation for primary gastric melanoma, a presentation that has been documented previously (2,3); other unique presentations of primary gastric melanoma include a non-healing ulcer with benign mucosa on initial biopsy (4), and progressive axilla swelling (18). Literature review of other cases of primary gastric melanoma and metastatic gastric melanoma reveals that the presentation is often vague with nonspecific symptoms of anorexia, dysphagia, nausea, vomiting, epigastric pain, fatigue, and weight loss (5-7,9,19,20). The vague symptoms and nonspecific resentation of gastric melanoma can lead to a delay in diagnosis.
There is still significant controversy surrounding even the diagnosis of primary malignant melanoma of the GI tract. Arguments in support of the idea that GI melanomas are metastatic lesions even in the absence of a primary are based on the natural history of melanoma. The fact that the GI tract is the most common site of metastases of cutaneous melanoma (21) and that the stomach epithelium is devoid of melanocytes is the foundational argument supporting the assertion that all gastric melanoma is metastatic (4,8). Additionally, several cases of spontaneous regression of a primary cutaneous melanoma with subsequent visceral and nodal metastases have been reported (22,23). An autopsy study on small bowel melanoma concluded that even in the absence of a known primary, small bowel melanoma most likely represents metastatic disease (24). Alternative explanations that argue for the development of primary GI melanomas include the migration of neural crest cells through the omphalomesenteric canal (an explanation that is applicable to melanoma of the ileum only) (25), and the neoplastic transformation of APUD cells (amine precursor uptake and decarboxylation cells) in noncutaneous sites (26,27).
The lack of clarity of GI melanoma pathogenesis has led to the development of criteria for diagnosing a primary GI malignant melanoma. These include: no concurrent or prior excision of melanoma or atypical melanotic lesion from the skin, lack of involvement of other organs, lack of in situ change in overlying or adjacent GI epithelium, and 12 month disease-free survival after diagnosis (28).
Management of primary gastric melanoma is primarily surgical. A review of nine cases of gastric melanoma in which no known extra-gastric primary was identified reveals that eight of the nine cases were treated with surgery. Three of the cases were treated with partial gastrectomy and splenectomy (2,4,6), two cases were treated with partial gastrectomy alone (5,8), one with total gastrectomy (7), one with gastrectomy, pancreatectomy, splenectomy, and transverse colectomy (9), and one stated to be “palliative resection” (3). Only one case was treated with adjuvant therapy and that patient received 12 months of adjuvant interferon (4). The primary gastric melanoma case that was not treated surgically was treated with dacarbazine and cisplatin-based chemo due to peripancreatic and axillary nodal metastases (18).
Those with no identifiable primary lesion had variable outcomes. In the case treated with partial gastrectomy and splenectomy followed by 12 months of adjuvant interferon, the patient showed no evidence of disease on EGD two years post-operative (4). Another case treated with partial gastrectomy and splenectomy showed a similar outcome with the patient being disease free at 16 months post-op (6), and one case reported patient survival with no evidence of disease at five years post-total gastrectomy (7). Of the surgical cases with poorer outcomes, one patient with comorbid dermatomyositis died due to post-operative complications following a partial gastrectomy (5), one patient succumbed to metastases 12 months following a distal gastrectomy (8), and another patient died 11 months post-operative following a gastrectomy, pancreatectomy, splenectomy, and transverse colectomy for a locally invasive gastric melanoma (9). Two cases were lost to follow up (2,3).
In contrast to the surgery-based management of gastric melanoma with no known primary, chemotherapy and radiation therapy play a larger role gastric melanoma with a known extra-gastric primary. One case of metastatic gastric melanoma was treated with a wedge resection of the cardia, but the patient ultimately underwent palliative whole brain radiation for recurrent metastases (29). In another case the patient received neoadjuvant temozolomide chemotherapy followed by a wedge resection of the stomach (29). Three other cases of metastatic gastric melanoma were managed with chemotherapy alone, one reported controlled disease after one course of dacarbazine, nimustine, and cisplatin (30), and two other reports did not state which chemotherapy agents were used (19,31).
Radiation therapy has been used to control bleeding in a variety of cancers. Studies have shown radiation therapy to be beneficial in controlling hemoptysis in lung cancer, hematuria in bladder cancer, and vaginal bleeding in cervical cancer (10-13), more recently studies on radiation therapy to treat gastric bleeding have been reported. One retrospective study demonstrated a 54% response to bleeding in patients with locally advanced or recurrent gastric cancer who were treated with radiation therapy alone (17). Another retrospective study demonstrated a 70% response to bleeding in patients who received radiation therapy with or without concurrent chemotherapy (16).
Subsequent studies have focused on the effects of radiation dose in symptomatic palliation. A 2009 retrospective study showed that patients with bleeding from primary gastric cancer who received a dose of greater than or equal to 40 Gy in 16 fractions have statistically significant improvement in control of bleeding compared to those who received less than 40 Gy in 16 fractions (15). Most recently a study on patients who received 30 Gy in 10 fractions showed a 73% hemostasis rate. Additionally this study demonstrated that those treated with chemotherapy and radiation had a significant longer time to rebleeding when compared to those who received radiation therapy alone (14).
The case presented marks the first use of standalone radiation therapy as a palliative therapy for persistent upper GI bleeding secondary to primary gastric melanoma. In the case presented, palliative radiation therapy of 16 Gy in four fractions provided four months of symptomatic relief. In addition, the patient tolerated a second course of therapy of 9 Gy in three fractions for his rebleeding and is currently asymptomatic.
In conclusion, malignant melanoma of the stomach with no identifiable extra-gastric primary is a rare occurrence with surgery being the current mainstay of therapy. In symptomatic patients who are poor surgical candidates, palliative radiation therapy can provide symptomatic relief and improve quality of life.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Patel JK, Didolkar MS, Pickren JW, et al. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg 1978;135:807-10. [PubMed]
- Noraidah M, Jasmi AY. Malignant melanoma of the gastrointestinal tract presenting as a bleeding gastric ulcer. Malays J Pathol 2003;25:57-61. [PubMed]
- Ravi A. Primary gastric melanoma: a rare cause of upper gastrointestinal bleeding. Gastroenterol Hepatol (N Y) 2008;4:795-7. [PubMed]
- Alazmi WM, Nehme OS, Regalado JJ, et al. Primary gastric melanoma presenting as a nonhealing ulcer. Gastrointest Endosc 2003;57:431-3. [PubMed]
- Castro C, Khan Y, Awasum M, et al. Case report: primary gastric melanoma in a patient with dermatomyositis. Am J Med Sci 2008;336:282-4. [PubMed]
- Lagoudianakis EE, Genetzakis MA, Papadima A, et al. Primary gastric melanoma: a case report. World J Gastroenterol 2006;12:4425-7. [PubMed]
- McKay JD, Deacon A. Isolated melanoma metastasis to stomach with possible regressed primary lesion: the importance of pursuing solitary melanoma metastases. N Z Med J 2010;123:78-9. [PubMed]
- Yamamura K, Kondo K, Moritani S. Primary malignant melanoma of the stomach: report of a case. Surg Today 2012;42:195-9. [PubMed]
- Yuan-Mou Yang J, Krishna GS, Macleod C, et al. Primary gastric mucosal melanoma. N Z Med J 2008;121:96-9. [PubMed]
- Biswal BM, Lal P, Rath GK, et al. Hemostatic radiotherapy in carcinoma of the uterine cervix. Int J Gynaecol Obstet 1995;50:281-5. [PubMed]
- Brundage MD, Bezjak A, Dixon P, et al. The role of palliative thoracic radiotherapy in non-small cell lung cancer. Can J Oncol 1996;6:25-32. [PubMed]
- Hanks G, Cherny NI, Christakis NA. eds. Oxford Textbook of Palliative Medicine. New York: Oxford University Press, 2004.
- Srinivasan V, Brown CH, Turner AG. A comparison of two radiotherapy regimens for the treatment of symptoms from advanced bladder cancer. Clin Oncol (R Coll Radiol) 1994;6:11-3. [PubMed]
- Asakura H, Hashimoto T, Harada H, et al. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol 2011;137:125-30. [PubMed]
- Hashimoto K, Mayahara H, Takashima A, et al. Palliative radiation therapy for hemorrhage of unresectable gastric cancer: a single institute experience. J Cancer Res Clin Oncol 2009;135:1117-23. [PubMed]
- Kim MM, Rana V, Janjan NA, et al. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol 2008;47:421-7. [PubMed]
- Tey J, Back MF, Shakespeare TP, et al. The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys 2007;67:385-8. [PubMed]
- Khaliq A, Siddappa PK, Thandassery RB, et al. Melanoma of stomach. J Gastrointest Cancer 2012;43:630-3. [PubMed]
- Bahat G, Saka B, Colak Y, et al. Metastatic gastric melanoma: a challenging diagnosis. Tumori 2010;96:496-7. [PubMed]
- Chehab BM, Dakhil SR, Nassif II. Melanoma metastatic to the duodenum presenting as upper GI bleed: 2 cases and a review of the literature. Gastrointest Endosc 2008;67:998-1000. [PubMed]
- Manouras A, Genetzakis M, Lagoudianakis E, et al. Malignant gastrointestinal melanomas of unknown origin: should it be considered primary? World J Gastroenterol 2007;13:4027-9. [PubMed]
- Ducic Y. Spontaneous regression of cutaneous melanoma with subsequent metastasis. J Oral Maxillofac Surg 2002;60:588-91. [PubMed]
- High WA, Stewart D, Wilbers CR, et al. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol 2005;53:89-100. [PubMed]
- Elsayed AM, Albahra M, Nzeako UC, et al. Malignant melanomas in the small intestine: a study of 103 patients. Am J Gastroenterol 1996;91:1001-6. [PubMed]
- Amar A, Jougon J, Edouard A, et al. Primary malignant melanoma of the small intestine. Gastroenterol Clin Biol 1992;16:365-7. [PubMed]
- Krausz MM, Ariel I, Behar AJ. Primary malignant melanoma of the small intestine and the APUD cell concept. J Surg Oncol 1978;10:283-8. [PubMed]
- Tabaie HA, Citta RJ, Gallo L, et al. Primary malignant melanoma of the small intestine: report of a case and discussion of the APUD cell concept. J Am Osteopath Assoc 1984;83:374-7. [PubMed]
- Sachs DL, Lowe L, Chang AE, et al. Do primary small intestinal melanomas exist? Report of a case. J Am Acad Dermatol 1999;41:1042-4. [PubMed]
- Liang KV, Sanderson SO, Nowakowski GS, et al. Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin Proc 2006;81:511-6. [PubMed]
- Matsubayashi H, Takizawa K, Nishide N, et al. Metastatic malignant melanoma of the gastric mucosa. Intern Med 2010;49:1243-4. [PubMed]
- Goral V, Ucmak F, Yildirim S, et al. Malignant melanoma of the stomach presenting in a woman: a case report. J Med Case Rep 2011;5:94. [PubMed]