Surgical and radiation therapy management of recurrent anal melanoma
Introduction
Malignant melanoma of the anorectal mucosa is a very rare but highly aggressive tumor. It constitutes less than 2% of all melanomas (1). Whether cutaneous or mucosal in origin, all melanomas originate from the embryologic neural crest-derived melanocyte cells. These melanocytes undergo malignant transformation to become melanoma. Increased exposure to sunlight, particularly ultraviolet B radiation and fair complexions are both well-documented risk factors for cutaneous melanoma (2). As such, it is unclear what triggers the development of anal melanoma since the anal canal is not exposed to sunlight. Other additional risk factors for cutaneous melanoma, such as xeroderma pigmentosum and dysplastic nevus syndrome, have no known association with anorectal melanoma. It is difficult to determine the precise disease incidence of anorectal melanoma given its relative rarity. However, a sampling of U.S. cancer registries revealed an incidence of 1.7 cases of anorectal melanoma per one million people per year (3). Anal melanoma most commonly presents in the sixth or seventh decade of life with some studies suggesting a slightly female predominance (2,4,5). Unlike cutaneous melanoma, there are no studies showing ethnic predilection with anal melanomas. Historically, surgery has been the mainstay of treatment. The most appropriate type of surgery, however, remains questionable as there is a lack of prospective or randomized data comparing local excisions with more radical surgeries. The role of adjuvant treatments is still in the process of being defined today. Nonetheless, anal melanoma carries a very poor prognosis. Median survival is less than two years despite curative treatment interventions (6). In many cases the tumor is already widely metastatic at the time of diagnosis (7). This often precludes attempts at definitive treatment. Despite the unpredictable nature of melanoma there are still instances of repeated local recurrence. Here, we present a patient who initially underwent surgical treatment for symptomatic hemorrhoids but was incidentally diagnosed with anal melanoma.
Case report
A 41-year-old Caucasian female with a history of rectal pain and hemorrhoids was referred to our hospital by her primary care physician for further evaluation. She first developed anal discomfort in 2011. She reported some discharge and weeping from the anorectal region. This was initially attributed to hemorrhoids. Her primary care physician noted a longstanding history of prolapsing internal and external hemorrhoids which were very symptomatic. She was seen by the colorectal surgeon at our hospital where an excisional hemorrhoidectomy was scheduled.
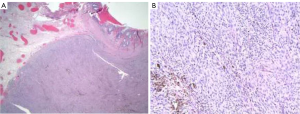
In the operating room, an anorectal exam was performed under general anesthesia. Inspection of the anorectal region showed a large right anterior prolapsing hemorrhoid strangulated in appearance. This led to an internal component with an adjacent smaller internal and external hemorrhoid. The hemorrhoidectomy was then performed with sphincter muscle preservation. Pathologic examination revealed an aggregate of hemorrhoids along with a pedunculated acutely eroded malignant melanoma with foci of junctional component highly suggestive of primary mucosal melanoma (Figure 1). The tumor measured 1.2 cm in thickness with an apparent 2 mm negative margin at the base.
Given this incidental diagnosis of mucosal melanoma a PET-CT of the whole body was performed as part of her metastatic workup. This showed a 1.4 cm × 1.3 cm enlarged right inguinal lymph node with increased FDG activity (SUV 4.0) which was highly suspicious for disease involvement. A core needle biopsy of this inguinal node done shortly thereafter confirmed metastatic melanoma. The patient was then referred to medical oncology who recommended tumor cytogenetic analysis. A right superficial groin lymph node dissection was also recommended and performed revealing one out of seven dissected lymph nodes positive for metastatic melanoma. B-Raf genotype testing was found to be negative. The use of systemic therapy, such as immunotherapy, was discussed with the patient but she was hesitant to undergo this treatment considering some of the possible side effects.
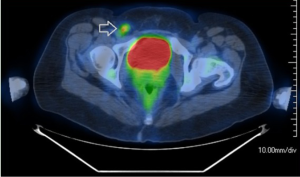
The patient continued to undergo routine surveillance postoperatively. A physical exam and PET-CT was performed every few months. Approximately seven months after her superficial groin lymph node dissection a routine surveillance PET-CT demonstrated a prominent right groin lymph node measuring 4.2 cm × 3.1 cm significantly larger compared to previous examination and now highly FDG-avid (SUV 19.5) (Figure 2). There was also a new soft tissue mass measuring 3.2 cm × 2.6 cm superior and lateral to the left aspect of the uterus showing increased FDG update (SUV 6.6). These two lesions were highly suspicious for recurrent disease (Figure 3).
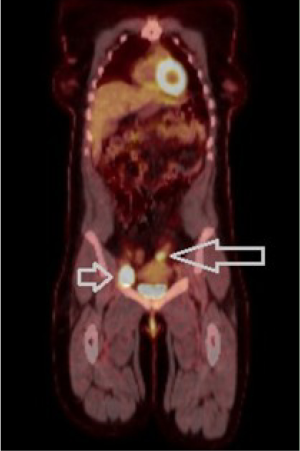
The patient’s case was discussed at tumor board where the recommendation was a right deep inguinal and pelvic lymph node dissection and full thickness resection of the recurrent rectal tumor. A diagnostic laparoscopy was performed prior to incision to verify no evidence of intra-abdominal metastatic disease. One surgeon performed an open right deep inguinal node and pelvic node dissection while a second surgeon simultaneously performed a transanal resection of the rectal tumor. A transanal local resection was chosen over a radical abdominoperineal resection (APR) given the lack of data demonstrating a long-term survival advantage with radical resection in this setting. Surgical findings showed a 3 cm anterior anorectal mass involving the rectovaginal septum. There was also a 1 cm right anterior satellite tumor within the sphincter muscle itself. This required vaginal wall placation and sphincteroplasty. Pathologic examination revealed a 2.2 cm mucosal melanoma with clear margins and a 1 cm melanoma satellite nodule with tumor cells seen at the inked margin. The enlarged right deep inguinal lymph node was positive for metastatic melanoma. The patient tolerated the surgery well and recovered without complications.
Medical oncology evaluated the patient again for the possibility of systemic therapy. The tumor was found to be B-Raf mutation negative but CDKN2A truncation mutation positive. The patient was referred to an outside medical oncologist for a second opinion and possible enrollment on a clinical trial. The patient decided to undergo Ipilumumab immunotherapy but was recommended to undergo adjuvant radiation therapy first. She was seen by radiation oncology and a course of hypofractionated radiation therapy was given. A dose of 48 Gy in 20 fractions was delivered over the course of four weeks using intensity-modulated radiation therapy to spare toxicity to surrounding organs at risk. The entire anal canal and regional lymph nodes, including internal and external iliacs, presacral, and inguinal nodes, were treated as the target volume.
During treatment the patient developed some expected skin erythema and desquamation. This was treated symptomatically with silvadene creme and sitz baths. She tolerated treatment well and was seen in follow-up one month after completing treatment. Her skin reaction healed and she denied any diarrhea, anorectal pain, nausea, rectal bleeding, or vaginal bleeding. She was then started on systemic Ipilumumab immunotherapy.
Discussion
It is not uncommon for patients with symptomatic anorectal melanoma to be misdiagnosed as having hemorrhoids. The most common presenting complaints include bleeding, anal mass, anal pain, tenesmus, and changes in bowel habit which are frequently shared with symptomatic hemorrhoids. On the other hand, systemic symptoms of weight loss and fatigue are typically seen only in the metastatic setting (8). There is often a delay in diagnosis of this disease for a number of reasons. First, lesions in the anorectum cannot be visualized by the patient. Many patients are aware of screening for cutaneous melanomas but these anorectal lesions simply cannot be seen. Patients also commonly report as much as a 4-6-month delay from symptom onset to presentation to their doctors (5). To complicate things further, it is reported that up to 20% of these tumors are histologically amelanotic and most lack even gross pigmentation (9). Lastly, as seen with the patient in this case report, symptoms of anorectal melanoma are frequently misdiagnosed as other more common anorectal etiologies such as hemorrhoids, polyps, or skin tags (10).
As a result of this delay in diagnosis, patients with anorectal melanoma often present with advanced disease. Symptomatic tumors are often greater than 1 cm thick at diagnosis with ulceration and lymph node involvement (11). The most common sites of nodal metastases are the inguinal lymph nodes, mesenteric lymph nodes, hypogastric lymph nodes, and para-aortic lymph nodes (8). Aside from thickness and lymph node involvement, other suggested negative prognostic indicators are duration of symptoms, tumor necrosis, perineural invasion, and the presence of amelanotic melanoma on histology (12). As such, a thorough diagnostic work-up including systemic imaging and endoscopic evaluation including endoscopic ultrasound is warranted if a diagnosis of anorectal melanoma is suspected.
Surgical resection is considered the mainstay of treatment for anorectal melanoma. However, controversy surrounding the optimal surgical management is a topic of ongoing study. Despite a lack of prospective or randomized data, there are generally two standard surgical approaches for this disease: a wide local excision (WLE) or a more extensive APR. Initially, APR was advocated in the setting of non-metastatic disease. Arguments favoring APR demonstrate the superior rates of local control which are achieved with a more extensive resection (13). Many of these patients are diagnosed at an advanced stage with either distant or extensive nodal involvement. In such cases an APR even with mesenteric dissection would not be curative (3,13,14). These patients tend to die from metastatic disease rather than local recurrences. This negates the local control benefit of radical resection. More recently, several study series have shown WLE to provide comparable survival outcomes with less peri-operative morbidities. Interestingly, WLE also served as curative surgery in some of these patients (7,11,15).
Surgical treatment of the surrounding lymph node areas remains a controversial topic. It was initially thought that lymph node dissection at the time of surgery was essential given the high rate of lymph node involvement with anorectal melanoma. It was thought that observation of lymph nodes until they were clinically suspicious would potentially miss a curative window of opportunity. However, several studies performed did not find a difference in overall survival with upfront mesenteric lymph node dissection. Higher rates of lymphedema and perioperative morbidity were seen with lymph node dissection (11,16). Despite attempts at curative surgery in patients with anorectal melanoma, the median survival is still dismal at less than 20 months (17). Accordingly, quality of life considerations must be taken into account. The surgical approach chosen should strive to find a balance between achieving local control and avoiding perioperative morbidity.
Disseminated metastatic disease is seen in as many as one third of anorectal melanoma patients at the time of disease presentation (18). The role of systemic therapy is not well established in this disease. Many agents have been employed in treating systemic melanoma. They include vincristine, dacarbazine, nimustine, cisplatin, and interferon. None of these have demonstrated a significant survival benefit in treating anorectal melanoma (19-21). The timing of systemic therapy is also unclear. Some advocate the use of systemic therapy in a palliative setting only while others advocate its use in the adjuvant setting. Biochemotherapy, a method of administering both a biologic and chemotherapeutic agent, has been used to successfully treat some cases of cutaneous melanoma (22). One series investigating biochemotherapy did show 44% good disease response which is higher than any documented individual chemotherapy series (23). Systemic interferon is another frequently used systemic therapy for melanoma. Interferon-α has shown antineoplastic effects related to a number of direct and indirect immune-modulating effects. One case study did demonstrate complete pathologic response of primary anorectal melanoma and near complete response of associated pulmonary metastases after combined interferon and dacarbazine administration (24). Data with systemic treatment is limited in the literature but these are encouraging findings which support further investigation into combined, multi-agent systemic therapies.
The role of radiation therapy in anorectal melanoma has largely been relegated to post-operative or palliative settings. One study demonstrated a local control rate similar to APR when radiation was given to the primary site after WLE. However, there was no difference in survival (25). A large Australian phase III randomized prospective trial investigated the role of adjuvant radiation therapy to clinically at-risk lymph node regions following lymph node dissection for nodal melanoma metastases. A hypofractionated regimen was used in this study and the risk of lymph node relapse was significantly decreased with adjuvant radiation therapy (26). This suggests a role for radiation therapy in subclinical disease. However, the patients in this trial had cutaneous melanoma and it is unclear whether these findings have any meaningful application to mucosal melanoma treated in the anorectal region.
Skin toxicities frequently cause breaks during the treatment course which may result in tumor cell repopulation and diminished treatment efficacy. Intensity modulated radiation therapy (IMRT) is a more modern technique of delivering radiation that allows sparing of surrounding structures at risk while escalating dose to the tumor. One prospective trial demonstrated a significant decrease in severe skin and gastrointestinal toxicity when treating anal tumors using IMRT (27). A decrease in severe treatment side effects may lead to less patient morbidity, fewer interruptions during treatment, and better local control. Improvements in treatment delivery techniques may pave the way for radiation to play a larger role in the treatment of anorectal melanoma.
In conclusion, anorectal melanoma is a rare but highly aggressive malignancy. Given the frequent delays in diagnosis many patients present with advanced or disseminated disease. Being a rare malignancy, there is a paucity of prospective and randomized studies. Surgery is considered the mainstay of treatment but the optimal surgical approach is still under debate. Many of these patients present with distant metastatic disease. Because of this, aggressive local surgeries may not be warranted since they demonstrate significant perioperative morbidity without improved survival outcomes. The roles of systemic and radiation therapy are still being defined. Combined systemic therapy with radiation therapy in addition to surgery will likely provide the best treatment outcomes for patients. The overall treatment goal should strive to optimize quality of life and tumor control while minimizing treatment-related morbidities.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Wanebo HJ, Woodruff JM, Farr GH, et al. Anorectal melanoma. Cancer 1981;47:1891-900. [PubMed]
- Bullard KM, Tuttle TM, Rothenberger DA, et al. Surgical therapy for anorectal melanoma. J Am Coll Surg 2003;196:206-11. [PubMed]
- Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum 1995;38:146-51. [PubMed]
- Roumen RM. Anorectal melanoma in The Netherlands: a report of 63 patients. Eur J Surg Oncol 1996;22:598-601. [PubMed]
- Pessaux P, Pocard M, Elias D, et al. Surgical management of primary anorectal melanoma. Br J Surg 2004;91:1183-7. [PubMed]
- Weinstock MA. Epidemiology and prognosis of anorectal melanoma. Gastroenterology 1993;104:174-8. [PubMed]
- Goldman S, Glimelius B, Påhlman L. Anorectal malignant melanoma in Sweden. Report of 49 patients. Dis Colon Rectum 1990;33:874-7. [PubMed]
- Petrelli NJ, Nagel S, Rodriguez-Bigas M, et al. Morbidity and mortality following abdominoperineal resection for rectal adenocarcinoma. Am Surg 1993;59:400-4. [PubMed]
- Slingluff CL Jr, Vollmer RT, Seigler HF. Anorectal melanoma: clinical characteristics and results of surgical management in twenty-four patients. Surgery 1990;107:1-9. [PubMed]
- Felz MW, Winburn GB, Kallab AM, et al. Anal melanoma: an aggressive malignancy masquerading as hemorrhoids. South Med J 2001;94:880-5. [PubMed]
- Cooper PH, Mills SE, Allen MS Jr. Malignant melanoma of the anus: report of 12 patients and analysis of 255 additional cases. Dis Colon Rectum 1982;25:693-703. [PubMed]
- Yeh JJ, Shia J, Hwu WJ, et al. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann Surg 2006;244:1012-7. [PubMed]
- Abbas JS, Karakousis CP, Holyoke ED. Anorectal melanoma: clinical features, recurrence and patient survival. Int Surg 1980;65:423-6. [PubMed]
- Pack GT, Lenson N, Gerber DM. Regional distribution of moles and melanomas. AMA Arch Surg 1952;65:862-70. [PubMed]
- Ross M, Pezzi C, Pezzi T, et al. Patterns of failure in anorectal melanoma. A guide to surgical therapy. Arch Surg 1990;125:313-6. [PubMed]
- Siegal B, Cohen D, Jacob ET. Surgical treatment of anorectal melanomas. Am J Surg 1983;146:336-8. [PubMed]
- Droesch JT, Flum DR, Mann GN. Wide local excision or abdominoperineal resection as the initial treatment for anorectal melanoma? Am J Surg 2005;189:446-9. [PubMed]
- Thibault C, Sagar P, Nivatvongs S, et al. Anorectal melanoma--an incurable disease? Dis Colon Rectum 1997;40:661-8. [PubMed]
- Nyui S, Osanai H, Masuoka H, et al. Anorectal malignant melanoma: report of a case. Surg Today 1997;27:753-6. [PubMed]
- Terada R, Ito S, Kobayashi M, et al. Anorectal melanoma: successful treatment by surgical excision and combination chemoimmunotherapy. Hepatogastroenterology 2002;49:1545-8. [PubMed]
- Veronesi U, Adamus J, Aubert C, et al. A randomized trial of adjuvant chemotherapy and immunotherapy in cutaneous melanoma. N Engl J Med 1982;307:913-6. [PubMed]
- Legha SS, Ring S, Bedikian A, et al. Treatment of metastatic melanoma with combined chemotherapy containing cisplatin, vinblastine and dacarbazine (CVD) and biotherapy using interleukin-2 and interferon-alpha. Ann Oncol 1996;7:827-35. [PubMed]
- Kim KB, Sanguino AM, Hodges C, et al. Biochemotherapy in patients with metastatic anorectal mucosal melanoma. Cancer 2004;100:1478-83. [PubMed]
- Ulmer A, Metzger S, Fierlbeck G. Successful palliation of stenosing anorectal melanoma by intratumoral injections with natural interferon-beta. Melanoma Res 2002;12:395-8. [PubMed]
- Konstadoulakis MM, Ricaniadis N, Walsh D, et al. Malignant melanoma of the anorectal region. J Surg Oncol 1995;58:118-20. [PubMed]
- Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol 2012;13:589-97. [PubMed]
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [PubMed]


