Utilization of intensity modulated radiation therapy for anal cancer in the United States
Introduction
Combined chemoradiotherapy (CRT) for anal cancer (AC) is an efficacious measure which provides high rates of disease control and survival; it is thus the consensus paradigm to treat most cases of AC (1). However, concurrent CRT can also incur high rates of treatment-related toxicities, including perianal symptoms, which can be severely debilitating and substantially impair tolerance of full-dose CRT as well as quality of life. To this extent, radiation therapy (RT) has markedly advanced from the historical use of two- or three-dimensional conformal RT (2D/3DCRT) to inverse-planned intensity-modulated RT (IMRT), which creates highly conformal dose distributions and minimizes toxicities to several organs-at-risk (OARs).
IMRT for AC has proven to reduce doses to multiple RT-sensitive OARs (e.g., external genitalia and bowel) as compared to 3DCRT (2-4). As such, multiple retrospective reports have shown IMRT to be safe and effective as part of a CRT regimen (5-11). However, prospective evidence for this is lacking, as just one trial has evaluated concurrent IMRT-based CRT to date; toxicity profiles were encouraging but did not meet its primary endpoint (12). As a result, both IMRT and 3DCRT are listed as recommended RT techniques in this circumstance (1).
In this study, the first of its kind to date, we sought to evaluate national trends of IMRT utilization as part of concurrent CRT for AC. We specifically evaluated temporal trends, along with factors associated with greater likelihood of IMRT delivery. Given the lack of prospective evidence and relatively low volume of retrospective data, this analysis of a large, contemporary national database has implications on payers and insurance coverage along with patient counseling.
Methods
This study analyzed the National Cancer Data Base (NCDB), which is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons (ACS) and the American Cancer Society. The NCDB consists of de-identified information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the US population (13-29). The NCDB contains information not included in the Surveillance, Epidemiology, and End Results (SEER) database, including details regarding use of systemic therapy. The data used in the study were derived from a de-identified NCDB file. The ACS and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
The most recently released NCDB dataset corresponded to the years 2004–2015. The inclusion criteria for this study involved patients age ≥18 with newly-diagnosed cT1–4 N0–3M0 anal comprising histologic codes of squamous cell carcinoma [International Classification of Disease for Oncology (ICD-O-3) codes 8051, 852, 8053, 8070-78, 8081, 8083-84, 8094, 8560, 8570]. For inclusion, patients required histological diagnostic confirmation and receipt of definitive concurrent CRT, defined as initiation of chemotherapy within 15 days of commencing RT. Since the purpose of the study was to compare the effect of radiation technique, inclusion criteria specifically involved the presence of a record of RT technique. Additionally, since our study sought to determine factors associated with use of IMRT, patients with unknown facility type or location were excluded. Using a classification scheme from other published studies utilizing the NCDB, an academic facility was an institution with both an accession of more than 500 newly diagnosed cancer cases per year and one that provided postgraduate medical education in at least four program areas, including internal medicine and general surgery (30). All other facilities, including Comprehensive Community Cancer Programs, Community Cancer Programs, and Integrated Network Programs, were categorized as non-academic, as none of these institutions require graduate medical education.
Information collected on each patient broadly included demographic data, comorbidity information, clinicopathologic tumor parameters, and treatment facility characteristics. All statistical tests were two-sided, with P<0.05 denoting statistical significance, and performed with STATA (version 14, College Station, TX, USA) software. The primary goal herein was to evaluate temporal trends and predictors of IMRT use. After baseline characteristics were compared between the IMRT and 3DCRT groups using χ2 or Fisher’s exact tests (non-parametric and parametric settings, respectively), multivariable logistic regression modeling was utilized to determine characteristics predictive for IMRT delivery. Overall survival (OS, defined as the interval between diagnosis and death, or censored at last contact) was not expected be different between groups and was thus investigated secondarily. The Kaplan-Meier method was used for survival analysis, and comparisons between IMRT and 3DCRT groups were performed with the log-rank test. Endpoints such as local control and cancer specific survival are not recorded in the NCDB. Patients with an unknown vital status were excluded from survival analysis. Univariate Cox proportional hazards modeling was additionally used to identify variables associated with OS, followed by multivariate analysis that included variables that were either significant or with a strong trend towards significance on univariate analysis.
Results
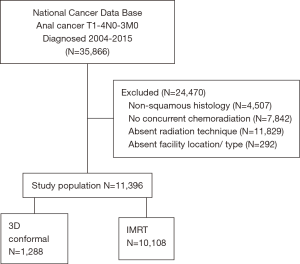
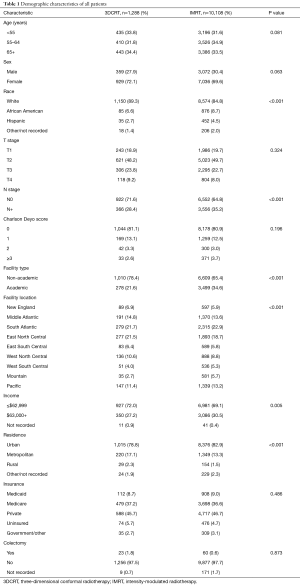
A complete flow diagram of patient selection is given in Figure 1. In total, 11,396 patients met study criteria (Table 1). Of these, 1,288 (11%) were treated with 3DCRT and 10,108 (89%) with IMRT. Analysis of temporal trends revealed a sharp rise in IMRT from 28% in 2004 to 96% in 2015 (Figure 2). This rise seemed to largely occur between 2004 and 2010; in the current decade, IMRT was delivered to >90% of patients without appreciable increase.
Full table
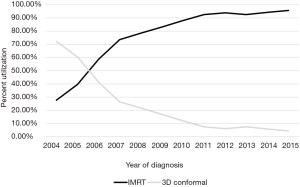
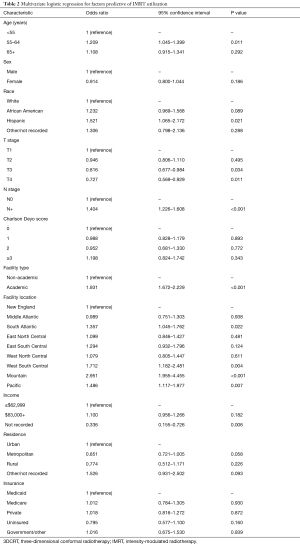
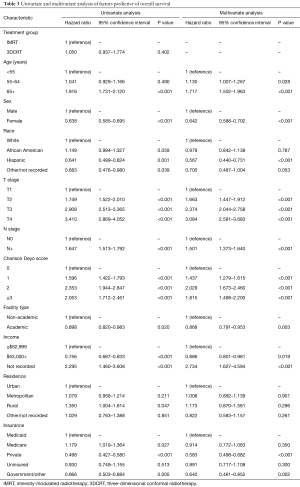
On multivariable logistic regression analysis (Table 2), patients treated with IMRT were more likely to be older (P=0.011) and Hispanic (P=0.021). IMRT was also more commonly delivered at academic centers (P<0.001). There were also regional differences; IMRT was more frequent in the South Atlantic (DC, DE, FL, GA, MD, NC, SC, VA, WV), West South Central (AR, LA, OK, TX), Mountain (AZ, CO, ID, MT, NM, NV, UT, WY), and Pacific (AK, CA, HI, OR, WA) regions (P<0.05 for all). Although IMRT was less likely administered for T3–4 disease (P<0.05 for both), it was more likely in node-positive cases (P<0.001). Of note, RT technique was not significantly impacted by patient insurance or income (P>0.05 for both).
Full table
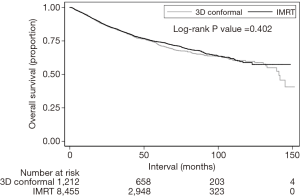
Median follow-up was 39.4 months (interquartile range, 23.2–62.5 months). Recognizing that OS was not expected to be different between groups, this analysis was performed secondarily. The 5- and 10-year OS for patients treated in the 3DCRT and IMRT groups were 72.0%/58.8% and 73.8%/59.7%, respectively (P=0.402) (Figure 3). In the overall cohort, there were several predictors of OS on Cox multivariate analysis (Table 3). These included advancing age, male gender, increasing comorbidities, advanced T stage, and node positivity (P<0.05 for all). Improved OS was observed in Hispanic patients, those treated at academic centers, and those with private/other governmental insurance (P<0.05 for all).
Full table
Discussion
Combined CRT for AC is difficult for patients to tolerate; to this extent, IMRT has proved to be a radiation technique associated with toxicity reduction. In this investigation, we demonstrate the sharp rise of IMRT from the mid-2000s to the mid-2010s. The large majority of patients are now treated with IMRT, with just a small minority still treated with 3DCRT. There were regional and disease-related factors associated with IMRT administration. Although RT technique did not impact OS as expected, academic centers were more likely to deliver IMRT, and OS was independently higher at these institutions.
The time course corresponding to the most rapid increase in IMRT utilization was between 2004 and 2010, with time points in the current decade showing consistently high (>90%) IMRT use. Although several pivotal papers (5,6) were published [and the RTOG 0529 trial commenced (12)] detailing the benefits of IMRT in this neoplasm between that time period, the findings herein likely correspond to a general increase in IMRT use during similar time periods owing to the adoption of new technology.
It is important to document that IMRT use was not independently linked with socioeconomic or insurance status, which would signal the necessity to address health disparities. Additionally, it is rather intuitive that IMRT was more often delivered for node-positive disease, given the clear need to treat pelvic and/or inguinal lymphatics (thus maximizing the therapeutic ratio for IMRT over 3DCRT). Although T3–4 disease was less likely treated with IMRT, which may seem counterintuitive, it is possible that these high-risk cases necessitated more urgent therapy (an advantage of forward-planned 3DCRT given the “effort” needed to generate a treatment plan), It is also possible that more of these patients were treated palliatively with lower doses, thus diminishing the value of IMRT over 3DCRT. To this extent, it is important to consider that the NCDB does not record information on whether a patient’s treatment plan was initially commenced with one technique and subsequently switched to another.
Regional differences were also appreciated between IMRT and 3DCRT usage, along with therapy at academic centers. Although it is clear that academic institutions are more at the forefront of newer therapies, modalities, and techniques, the independently finding of improved OS at these facilities has far-reaching implications on patient counseling and management by both oncologists and referring providers. These findings are in accord with data from other neoplasms demonstrating improved outcomes at academic and/or high-volume facilities (31,32). There are several potential reasons for this, not limited to greater multimodality coordination, streamlined and thorough diagnostic processes, technical expertise, ancillary support staff for close clinical monitoring, and potentially the availability of salvage therapies (or clinical trials). Nevertheless, this finding may impact any case of nonmetastatic AC and could warrant revisions in patterns of patient education.
In light of these data together with the aforementioned lack of further prospective work regarding this topic, it is important to appraise the cost-effectiveness of IMRT in this clinical circumstance. Two studies of cost have been performed using the SEER-Medicare database, demonstrating higher base costs for IMRT but decreased hospitalization-related costs (33,34). Presently, however, because neither of these “cost” studies were comparative “cost-effectiveness” studies, this question remains unresolved to date with respect to formal medico-economic analyses. Nevertheless, this work demonstrates the high rate of IMRT utilization across the nation, which also was not impacted by insurance or socioeconomic status; this may assist in indirectly speaking to this issue.
Although the NCDB provides a unique platform with which to study this important clinical question, limitations cannot go unacknowledged. First, NCDB investigations are inherently retrospective and can never eliminate selection biases, including referral patterns, judgment by individual providers, and nature of follow-up management. Second, although the NCDB encompasses roughly 70% of the US population, only CoC-accredited centers contribute data. Thus, the findings may not necessarily be representative of the entire US and international population, including countries without access to advanced technologies. Third, there was no specific dose-based cutoff in this study, which as mentioned above may have resulted in the inclusion of some palliatively-treated 3DCRT patients and skew the data accordingly (however, there were no statistical differences in OS). Fourth, the inclusion of T1N0 patients (albeit a very small percentage) may also skew the aforementioned figures as well. Lastly, the NCDB does not keep track of several noteworthy variables, such as HIV status, RT field design/volumes, specific chemotherapy type, or other endpoints such as tolerance of therapy (including premature cessation of chemotherapy and/or RT).
Conclusions
From this study of a large, contemporary national database, we demonstrate that the large majority of AC patients are now treated with IMRT, with a small minority still treated with 3DCRT. Regional and disease-related factors associated with IMRT administration are described. Although RT technique did not impact OS as expected, academic centers were more likely to deliver IMRT, and OS was independently higher at these institutions. Collectively, these data have notable implications for multidisciplinary oncologic providers, payers and insurance coverage, in addition to patient counseling by both referring and treating clinicians.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This study was exempt from institutional review board since study used de-identified patient information from NCDB.
References
- National Comprehensive Cancer Network. Anal Carcinoma. Version 4. 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf. Accessed November 26, 2017.
- Chen YJ, Liu A, Tsai PT, et al. Organ sparing by conformal avoidance intensity-modulated radiation therapy for anal cancer: dosimetric evaluation of coverage of pelvis and inguinal/femoral nodes. Int J Radiat Oncol Biol Phys 2005;63:274-81. [Crossref] [PubMed]
- Arbea L, Ramos LI, Martinez-Monge R, et al. Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol 2010;5:17. [Crossref] [PubMed]
- Sale C, Moloney P, Mathlum M. Carcinoma of the anal canal: Intensity modulated radiation therapy (IMRT) versus three-dimensional conformal radiation therapy (3DCRT). J Med Radiat Sci 2013;60:145-55. [Crossref] [PubMed]
- Milano MT, Jani AB, Farrey KJ, et al. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2005;63:354-61. [Crossref] [PubMed]
- Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol 2007;25:4581-6. [Crossref] [PubMed]
- Bazan JG, Hara W, Hsu A, et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011;117:3342-51. [Crossref] [PubMed]
- DeFoe SG, Beriwal S, Jones H, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal carcinoma--clinical outcomes in a large National Cancer Institute-designated integrated cancer centre network. Clin Oncol (R Coll Radiol) 2012;24:424-31. [Crossref] [PubMed]
- Chuong MD, Freilich JM, Hoffe SE, et al. Intensity-Modulated Radiation Therapy vs. 3D Conformal Radiation Therapy for Squamous Cell Carcinoma of the Anal Canal. Gastrointest Cancer Res 2013;6:39-45. [PubMed]
- Mitchell MP, Abboud M, Eng C, et al. Intensity-modulated radiation therapy with concurrent chemotherapy for anal cancer: outcomes and toxicity. Am J Clin Oncol 2014;37:461-6. [Crossref] [PubMed]
- Call JA, Prendergast BM, Jensen LG, et al. Intensity-modulated Radiation Therapy for Anal Cancer: Results From a Multi-Institutional Retrospective Cohort Study. Am J Clin Oncol 2016;39:8-12. [Crossref] [PubMed]
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: A Phase 2 Evaluation of Dose-Painted Intensity Modulated Radiation Therapy in Combination With 5-Fluorouracil and Mitomycin-C for the Reduction of Acute Morbidity in Carcinoma of the Anal Canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [Crossref] [PubMed]
- Bilimoria KY, Stewart A, Winchester D. at al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Bott MJ, Patel AP, Verma V, et al. Patterns of care in hilar node-positive (N1) non-small cell lung cancer: A missed treatment opportunity? J Thorac Cardiovasc Surg 2016;151:1549-58.e2. [Crossref] [PubMed]
- Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer 2017;103:11-6. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol 2017;133:369-75. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. National practice patterns and outcomes for T4b urothelial cancer of the bladder. Clin Genitourin Cancer 2017. [Epub ahead of print]. [PubMed]
- Moreno AC, Verma V, Hofstetter WL, et al. Patterns of care and treatment outcomes of elderly patients with stage I esophageal cancer: analysis of the National Cancer Data Base. J Thorac Oncol 2017;12:1152-60. [Crossref] [PubMed]
- McMillan MT, Ojerholm E, Verma V, et al. Radiation Treatment Time and Overall Survival in Locally Advanced Non-small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;98:1142-52. [Crossref] [PubMed]
- Verma V, Ryckman JM, Simone CB 2nd, et al. Patterns of care and outcomes with the addition of chemotherapy to radiation therapy for stage I nasopharyngeal cancer. Acta Oncol 2018;57:257-61. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. National Cancer Data Base Report on Pneumonectomy Versus Lung-Sparing Surgery for Malignant Pleural Mesothelioma. J Thorac Oncol 2017;12:1704-14. [Crossref] [PubMed]
- Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: Analysis of the National Cancer Data Base. Gynecol Oncol 2017;144:28-33. [Crossref] [PubMed]
- Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;97:146-54. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Radical cystectomy versus chemoradiation for musle-invasive bladder cancer: impact of treatment facility and sociodemographics. Anticancer Res 2017;37:5603-8. [PubMed]
- Haque W, Verma V, Butler EB, et al. Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol 2018;9:80-9. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck 2018;40:696-706. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Addition of chemotherapy to hypofractionated radiotherapy for glioblastoma: practice patterns, outcomes, and predictors of survival. J Neurooncol 2018;136:307-15. [Crossref] [PubMed]
- Verma V, Allen PK, Simone CB 2nd, et al. Addition of definitive radiotherapy to chemotherapy in patients with newly diagnosed metastatic nasopharyngeal cancer. J Natl Compr Canc Netw 2017;15:1383-91. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Chemotherapy versus chemoradiation for node-positive bladder cancer: practice patterns and outcomes from the National Cancer Data Base. Bladder Cancer 2017;3:283-91. [Crossref] [PubMed]
- Brower JV, Chen S, Bassetti MF, et al. Radiation dose escalation in esophageal cancer revisited: a contemporary analysis of the National Cancer Data Base. Int J Radiat Oncol Biol Phys 2016;96:985-93. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol 2017;135:173-81. [Crossref] [PubMed]
- Verma V, Allen PK, Simone CB 2nd, et al. Association of Treatment at High-Volume Facilities With Survival in Patients Receiving Chemoradiotherapy for Nasopharyngeal Cancer. JAMA Otolaryngol Head Neck Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Pollom EL, Wang G, Harris JP, et al. The Impact of Intensity Modulated Radiation Therapy on Hospitalization Outcomes in the SEER-Medicare Population With Anal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys 2017;98:177-85. [Crossref] [PubMed]
- Chin AL, Pollom EL, Qian Y, et al. Impact of Intensity-Modulated Radiotherapy on Health Care Costs of Patients With Anal Squamous Cell Carcinoma. J Oncol Pract 2017;13:e992-1001. [Crossref] [PubMed]