Uncovering the barriers to undergoing screening among first degree relatives of colorectal cancer patients: a review of qualitative literature
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide (1,2). Screening for CRC has always been recommended by various health organizations as it allows removal of adenomatous polyps, the precursors of CRC, and also enables earlier detection and treatment of early stage cancers (3-5). Whilst many postulate the pathogenesis of CRC to be due to undesirable lifestyle factors, the impact of familial predisposition is often underemphasized (6,7). Many guidelines across the world, including the one from the Ministry of Health, Singapore, have identified first degree relatives (FDR) of patients with CRC as an “increased-risk” group for screening after several studies have demonstrated higher rates of advanced adenomatous polyps and cancers amongst FDRs compared to the general population (6-14). However, screening rates amongst the FDRs remained abysmal (9-14).
Numerous qualitative studies have reported the lack of knowledge, perceptions and barriers of CRC screening amongst the general population (15-19). Interestingly, very few qualitative studies to uncover the barriers and other issues surrounding CRC screening from the perspectives of the FDRs of CRC patients have been conducted (20-24). In addition, for any screening program involving cancer patients and their family members to be successful, the role of healthcare professionals such as general practitioners (GPs) and specialists cannot be neglected. Active engagement and counseling by the healthcare professionals have been shown to result in higher compliance rates for CRC screening (25,26). However, it is apparent that several barriers are also encountered by the healthcare professionals which hinder them to advocate CRC screening among FDRs of CRC patients (27-29).
Hence, in light of the limited understanding of the issues on CRC screening among FDRs of CRC patients, we undertook a systematic review to achieve a better understanding of the pertinent challenges surrounding CRC screening amongst the FDRs and healthcare professionals’ perspectives.
Methods
Our review was based on the methodological framework as described by Arksey and O’Malley (30-33). This translates to (I) identification of the research question; (II) comprehensive search of the literature; (III) selection of the study based on inclusion and exclusion criteria; (IV) charting the data; and (V) collating, summarizing and reporting the results (30).
The key research questions for this review included:
- What are the barriers faced by the FDRs of CRC patients in undergoing screening for CRC?
- What are the barriers faced by healthcare professionals in advocating screening amongst the FDRs of CRC patients?
Search strategy
A comprehensive search of the literature published from January 2000 till February 2017 was conducted using databases which included PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), SCOPUS, and PsycINFO. The concepts searched were pertinent to the topics of CRCs, family members and screening. We used MeSH headings and free text key words combined with Boolean operators (Colorectal cancer OR Colon Cancer OR Rectal Cancer) AND (Screening or Prevention), AND (Family OR Relatives OR Siblings OR Children OR Parents) AND (Barriers OR Knowledge OR Attitude OR Perception OR Issues). This search was supplemented with manual searches of the reference lists of extracted articles. The keywords were intentionally broad-based to avoid exclusion of any relevant studies during the initial review.
Study selection
Studies were included if they were (I) qualitative in nature; (II) focusing on adult FDRs of CRC patients; (III) published; (IV) English and non-English; and (V) pertaining to topic on screening for CRC. Studies were excluded if they were (I) review articles; (II) quantitative studies; (III) limited to genetic testing; (IV) not related to screening; (V) not related to CRC and (VI) interventional studies.
Data extraction and analysis
A standardized data spreadsheet was used to chart the data from the articles. The data collected included each study’s design, country of study, study population, setting, objectives and results. Table 1 highlighted the shortlisted articles used for this review. The selected studies were reviewed and the key points were extracted and organized accordingly. As there were only eight studies included in this scoping review, we decided not to use NVivo software to help with the analysis. To help with identifying the themes, related to the CRC barriers from the FDRs’ and health professionals’ perspectives, thematic analysis was conducted to code meaningful statements from the results of the qualitative studies (34). The codes that emerged were then groups into themes which were reviewed and agreed by the team. Themes were then identified and analyzed and commonalities across the studies were reported accordingly using a narrative approach.

Full table
Quality appraisal of studies
These qualitative studies were assessed based on the 32-item consolidated criteria for reporting qualitative research (COREQ) checklist described by Tong et al. (35). The checklist evaluated three domains that enabled adequate evaluation of the quality of each study. These include: Research team and reflexivity; Study design; and Analysis and findings. The quality of the studies was deemed to be satisfactory with some deficiencies reported in the study design and analysis and findings domains. However, we continued to interpret the findings from these studies due to the already limited number of publications in this field.
Results
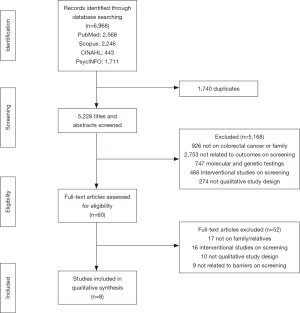
A total of 6,968 records were first identified and following exclusion of the duplicates, 5,228 articles were evaluated. After going through the titles and the abstracts, a total of 5,168 were excluded due to criteria as described prior. The remaining 60 full text articles were reviewed with emphasis on the study’s methodology, objectives and results. Ultimately, eight articles were included in the final review (20-24,27-29). Four articles originated from the United States of America (20-24), followed by France (27,29) and United Kingdom (28). Figure 1 illustrates the flow of our review process.
Our findings highlighted several themes for the barriers with regards to CRC screening from FDRs and healthcare professionals’ perspectives. The specific themes from the FDRs and healthcare professionals are described below.
Barriers from FDRs’ perspectives
Fear of diagnosis of cancer
The fear of the diagnosis of cancer from the screening tests often drove the participants away from undergoing screening in the first place. They were also worried that the diagnosis of cancer would lead to imminent death. This fear then resulted in the “belief” that not finding out the diagnosis would translate to the absence of disease: “People are afraid that they going to find something wrong.” (20), “I go to the doctor—the other day I was feeling kind of bad. I don’t know what that was but I’m afraid to go, afraid they going to find out what it is. I’m afraid that the doctor will tell me I am not going to be around much longer, so if I’m going to go, let me go.” (20), “People are fearful of what they might hear. They think a positive DRE is the tip of the iceberg and don’t want to know the rest of the story.” (24); “Positive results from flexible sigmoidoscopy would mean more anxiety, panic.” (24).
Negative attitude towards screening tests
FDRs often feel uncomfortable or embarrassed of undergoing the invasive endoscopic procedure. The perceived discomfort, embarrassment and feeling of being “violated” are strong emotions that were elicited: “It’s going to hurt/the prep is unpleasant/I’ve heard awful stories from others” (23); “and then the pain associated with the camera and tube going up, and things like that, so I mean, these are little things that turned me off with the idea.” (20); “I one time had that particular test (sigmoidoscopy) and I thought it was more embarrassing than painful” (22). In addition, the cost of the procedure was another factor that was mentioned: “But I haven’t heard anything about anything free, I haven’t checked with my doctor or anything like that, you know, my insurance would cover it, you know, I’m not sure,” (20); “For many of us here, asking for help is hard to do. I was raised to take care of myself, so having cancer would be hard for me because I take care of myself” (22). Some participants were also apprehensive regarding the accuracy and efficacy of the screening tests: “I was a little apprehensive, you know, about getting checked. I wasn’t sure it would detect cancer. That was one of the experiences.” (20); “I would always like to know, what is the reliability of the test. Once I take this test, does it tell me I am cancer free, or is there a 50% chance of the test being inaccurate.” (24); If flexible sigmoidoscopy examines only half (of the colon), what if the problem is in the other half?” (24); “I think the (colonoscopy) would be uncomfortable. I don’t know that it would necessarily be painful, but uncomfortable, yes.” (24).
Lack of awareness
FDRs were not aware of being at higher risk than the average population of developing CRC as they did not feel different from the other individuals who do not have a family history of CRC: “I found out that I did have polyps because the doctor said, “Hey, your brother’s got cancer, maybe you ought to go and get treated,” and they found one big one in there and they said, “This thing has been there for a lot of years, but luckily it wasn’t cancerous.” (20) On the other hand, because of the experience faced by the patient during the treatment, some of the FDRs were keen to be ignorant of the entire situation by not finding out more about the disease and screening tests “You don’t hear very much about these types of tests. I really couldn’t tell you” (22); “I guess I’ve been putting it off, too, so now I say I’m going to get some more information.” (20). Many also felt that investigations should only be performed in the presence of symptoms as they were unaware that CRC and its precursors could occur in the absence of symptoms: “I have no symptoms/I feel fine” (23); and lastly, the lack of accurate information amongst the community also influenced the behaviors of the FDRs: “But hearing it from hearsay, or people that have no idea what it is, sort of frightens people away.” (20); “You’ve got so many things on your mind and if you’re working and you’ve got kids, you’re not going to think about having a flexible sigmoidoscopy unless something gets your attention.” (24); “It’s not ever on TV. Like it probably should be. It would make us more comfortable as a culture if it was more widely discussed and we were educated.”
Locus of control
Whilst some participants adopted a fatalistic approach towards CRC, others felt that they were immune to the disease entity entirely and hence no action was necessary to address a problem that would not happen: “I largely believe that (I) don’t have any control of it” (21); “Just bad luck, that’s life.” (27); Some even believed that by changing their lifestyle entirely following the diagnosis of CRC would enable the body to heal on its own and reverse the process without medical treatment; “I know I’m going to die someday. In the event that I begin to feel symptoms, I’ll go on a complete vegetable and fruit diet. I believe my body can cleanse itself if I go back to nature and let nature do its job” (22).
Cultural factors
Unfortunately, there were some participants who had lost confidence in their healthcare professionals and system and hence were suspicious of their advice for screening or further investigation: “My mother had it and she went over to some quack doctor. Her first surgery, I believe they said it wasn’t necessary” (22). Some also mentioned that it is a taboo to discuss about cancer in their community and this prevented the message of screening being advocated in the family: “Nobody in my family ever talked about it. You know how old folks are—never talked about it before,” (20) “When I’ve mentioned colon cancer to my other doctors, they implied it wasn’t a big deal. I think with our family history, I want to know more about this and some doctor’s attitudes were, “Eh, don’t worry about that.” (24).
Barriers from physicians’ perspectives
Breach of patient’s confidentiality
Several physicians highlighted that patients are the best advocates for screening amongst the family members and they are hesitant to go against patient’s wishes, with the potential to breach patient’s confidentiality by doing so. “We can’t go against the patient’s wishes by informing his FDRs directly. That would be a breach of medical confidentiality.” (27); The physicians felt that it would not be ideal if they are the ones broaching the topic without the consent of the patients. “We must succeed in persuading the patient to inform his family” (29); “We must tell him to pass on the information” (29); “The patient is the cornerstone of his case” (29).
Lack of ownership
Several physicians felt that other parties, but not themselves, should be advising the FDRs instead. Several also didn’t feel the need to be involved and hence were not motivated to initiate the discussion with FDRs: “I need to be motivated myself if I am to motivate others. That’s the crux of the matter.” (29); A centralized cancer programme has been proposed to monitor the scheduling of screening colonoscopy amongst patients and FDRs; “We need a proper national cancer programme, otherwise the patients fall through the net.” (28); “A structure outside the patient-caregiver relationship, such as a care network, could take responsibility for contacting siblings and informing them about CRC screening.” (29), “We don’t want to obsess and medicalise our patients when we have quite a lot of suspicion that there is not going to be much that can be offered by the experts to reassure them.” (28). Moreover, other physicians felt uncomfortable advocating a procedure that is associated with inherent risks, especially when FDRs were asymptomatic and they are not the ones performing the procedure. “It (colonoscopy) is invasive and is potentially harmful” (28); “We inflict an examination on someone who has no symptoms but is aware of the risks and drawbacks, especially as we are obliged to mention the risk of perforation even though it’s very low.” (29).
Constraints of healthcare providers
Many physicians also cited time and resource constraints from their daily workload as the barriers. The need to uncover the family history of CRC amongst other cancers from individuals who seek medical treatment at their clinics was tedious and will take up significant amount of time: “I have enough trouble managing my clinical workload.” (29); “if we do all that here, take detailed family histories and determine risk, and then we find someone who’s at risk, then we are still going to send them up to secondary care, and that’s massive duplication of work, and I think you have to be careful about that.” (28); “The patient does not necessarily volunteer this information. And it’s true that one doesn’t take the time at the first consultation to take a family history.” (29).
Lack of (accurate) information from the FDRs
On the other hand, other physicians felt that it could be difficult to gather accurate information on the family history of CRC from FDRs. In addition, several physicians also pointed out that the relationship between family members can be estranged and this would lead to inaccurate information being relayed: “Often patients can’t tell us if their ill relative was diagnosed at 40, 50 or 60 years of age; and is it really necessary to be accurate to 5 years?” (29); “Families may have fallen out with one or other set of parents and no longer be on speaking terms; they might find it especially difficult to pass on information that cuts both ways—yes, it can save lives, but it’s also a question of cancer and death.” (29).
Inadequate or overload of information
The studies also highlighted that physicians are facing an arduous task of interpreting the burgeoning amount of information that is ever present, which could either mislead FDRs or even physicians themselves. They were either not updated on the latest guidelines or got confused from the excessive amount of information available: “We need a wider range of patient education materials.” (29) “We need the information. It doesn’t say you have to refer or anything, it’s just information so we can have it at our fingertips.” (28) “I don’t think it is very easy or possible at all maybe, to communicate concepts like high risk, low risk to everybody equally. Everybody has got a different idea: you run the risk of doing a lot of harm by saying ‘You are at moderate risk of bowel cancer’, which might be very low comparative to all the other risks that are out there.”(28) “The rules are less clear than those for breast cancer.” (29); “We receive too many recommendations.” (29).
Discussion
To our knowledge, this review is the first to comprehensively review the barriers faced by FDRs regarding the need for CRC screening. Apart from the perspectives of the FDRs, the viewpoints from the healthcare providers also lend further insight into the issue. From our review, several main overarching themes emerged from the thematic analysis that would be useful for healthcare professionals and policy makers to consider deriving interventions to address this matter.
From the FDRs’ perspectives, the lack of awareness of their familial predisposition of developing CRC and their negative attitudes towards the screening tests should probably be targeted first. It is perhaps not surprising that the FDRs were not cognizant of their “increased risks” due to several reasons. Firstly, the focus of any government-linked health agency is to improve the screening rates for all cancers across the entire population. It is already difficult for them to get more of the general population to undergo screening for the various cancers (36,37). If we were to focus the issue on CRC alone, any policy maker would be hesitant to further stress the difference of the risks between the average risk general population and the FDRs who are regarded as “increased risk” individuals. This might further confuse the general population more and deter them from undergoing CRC screening.
At the same time, it is difficult to dispel the issues pertaining to an endoscopic evaluation of the colon and rectum easily (38-41). The exhausting bowel preparation, associated with the inherent, albeit extremely small, risks of the procedure, coupled with the other issues such as cost and accessibility of the procedure only make the procedure less desirable (38-41). In addition, the “feeling of being violated” and the embarrassment associated with exposing a delicate part of the body to the endoscopist only made them more apprehensive about the procedure.
The authors believe that one of the most direct ways to counsel and educate these FDRs is through the patients themselves with the support and advice from the healthcare professionals. It has been shown that active intervention and counseling from the family practitioners have led to higher compliance rates for CRC screening (25,26). Moreover, when we focused our care on the patients with CRC, many other specialists are often involved and everyone should play an important role in advocating the importance of screening amongst the accompanying family members. Patients themselves are perhaps the best advocates to emphasize on the importance of screening amongst their family members following their own experiences and ordeals in overcoming the disease. The active engagement between patients and their FDRs on this topic could be a facilitating factor in increasing compliance to screening amongst their FDRs (27-29). The patients themselves must be firstly educated by the various healthcare professionals of the inherent increased risks that are present among their family members.
Based on the earlier discussion, it may be easy to criticize the physicians for not doing more to promote screening amongst the FDRs. However, our scoping review has identified some of the barriers they faced. Moreover, physicians also felt that the CRC patients are best positioned to advise their FDRs and loved ones on the benefits of screening and that it is tricky and a potential breach of confidentiality by advocating to their FDRs directly against the consent of the patients. This is especially so if the patients with CRCs are the physicians of the patients and not of the FDRs.
For the general practitioners, it is also difficult to bring forth the topic of screening amongst their patients especially if the individuals are consulting them for non-related minor ailments. Furthermore, the constraints posed by clinical workload also hinder the keenness of the doctors to further delve into the topic of family history and the various recommendations for cancer screening (42-44). Whilst it may appear to be unforgiveable to the specialists that general physicians are not up to date with the latest guidelines on screening for the various types of cancers, we need to realize that the ever-burgeoning literature and guidelines can only bog the family practitioners down. This has prompted some physicians to suggest creation of a central web-based cancer resource centre to be created to address any queries whenever present (27-29).
In addition, some physicians were also keen for the local public health agency to drive and be in charge of tackling the issue of cancer screening actively (27-29). The proposal includes a central agency to capture, monitor and recall individuals who are due for surveillance colonoscopy. Although this sounds logical and doable, the extent of logistics and manpower resources that is required to undertake this endeavor would be considerable. Apart from CRC, other stakeholders would also feel that many other conditions are just as, if not more, suitable to benefit from such a system. The prioritization of the conditions to target and the actual execution would be a mammoth undertaking.
One of our study’s main limitations was whether relevant studies could have been left out despite our structured and extensive search of the literature to identify suitable studies that fulfilled the inclusion and exclusion criteria. Moreover, we were only able to harness the information based on eight qualitative studies for this review, despite our exhaustive undertaking. Furthermore, our interpretation of the themes may have been influenced by the personal views of the reviewers. We attempted to minimize this potential bias by adhering to the framework as proposed by Arksey and O’Malley and by continuously engaging in team discussion to interpret the findings. That said, our review highlighted several barriers that have been under emphasized previously in an important yet neglected group of individuals. This can be seen by the significant differences in the extent of literature between FDRs and the general population.
Moving forward, more work is needed to ultimately increase the screening rates amongst the FDRs of CRC patients. Firstly, there is the need to validate the findings in the local context outside Europe and the USA as the reviewed studies were only from these countries. The differences in the healthcare system, values and socio-cultural dynamics amongst the communities must be borne in mind before considering and eventually implementing any intervention. Nevertheless, the authors felt that the identified barriers and associated factors will assist healthcare policy makers to gain further insight on the best methods to minimize these barriers and to improve the screening uptake amongst the FDRs. One of our recommendations is to explore the possibility of actively engaging patients to be advocates for CRC screening amongst their family members. The various healthcare providers must also play an active role in identifying opportunities to relate the important message of CRC screening to the patients or directly to FDRs. There is also a need for targeted interventions to overcoming the other barriers identified by the FDRs and healthcare providers.
Conclusions and implications
A lack of awareness of being at higher risks of developing CRC coupled with negative attitude towards colonoscopy were the main barriers faced by FDRs of CRC patients. Healthcare providers are more comfortable with patients being the advocates of CRC screening among their FDRs as they face other barriers and constraints in advocating screening among FDRs. Our review provides a conceptual framework to guide further research into the necessary actions and interventions with the ultimate aim of improving CRC screening participation rates amongst this increased risk group of individuals.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ministry of Health HPB, National Registry of Disease Office. Singapore Cancer Registry Interim Annual Registry Report, Trends in Cancer Incidence in Singapore2009-2013. Available online: https://www.nrdo.gov.sg/docs/librariesprovider3/Publications-Cancer/trends-in-cancer-incidence-in-singapore-2009-2013-interim.pdf?sfvrsn=0
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683-91. [Crossref] [PubMed]
- Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev 2007.CD001216. [PubMed]
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;315:2576-94. [Crossref] [PubMed]
- Winawer SJ. Screening of colorectal cancer. Surg Oncol Clin N Am 2005;14:699-722. [Crossref] [PubMed]
- Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 2001;96:2992-3003. [Crossref] [PubMed]
- Samadder NJ, Curtin K, Tuohy TM, et al. Increased risk of colorectal neoplasia among family members of patients with colorectal cancer: a population-based study in Utah. Gastroenterology 2014;147:814-21.e5; quiz e15-6.
- Lee HP, Chew CT, Consigliere DT, et al. Ministry of health clinical practice guidelines: cancer screening. Singapore Med J 2010;51:170-3. [PubMed]
- Samadder NJ, Smith KR, Mineau GP, et al. Familial colorectal cancer risk by subsite of primary cancer: a population-based study in Utah. Aliment Pharmacol Ther 2015;41:573-80. [Crossref] [PubMed]
- Puente Gutiérrez JJ, Marin Moreno MA, Dominguez Jimenez JL, et al. Effectiveness of a colonoscopic screening programme in first-degree relatives of patients with colorectal cancer. Colorectal Dis 2011;13:e145-53. [Crossref] [PubMed]
- Hogan NM, Hanley M, Hogan AM, et al. Awareness and uptake of family screening in patients diagnosed with colorectal cancer at a young age. Gastroenterol Res Pract 2015;2015:194931. [PubMed]
- Koc S, Esin MN. Screening behaviors, health beliefs, and related factors of first-degree relatives of colorectal cancer patients with ongoing treatment in Turkey. Cancer Nurs 2014;37:E51-60. [Crossref] [PubMed]
- Sassoli de Bianchi P, Campari C, Mancini S, et al. Colonoscopic surveillance of first-degree relatives of colorectal cancer patients in a faecal occult blood screening programme. Cancer Epidemiol 2013;37:469-73. [Crossref] [PubMed]
- Cha JM, Lee JI, Joo KR, et al. First-degree relatives of colorectal cancer patients are likely to show advanced colorectal neoplasia despite a negative fecal immunochemical test. Digestion 2012;86:283-7. [Crossref] [PubMed]
- Honein-AbouHaidar GN, Kastner M, Vuong V, et al. Systematic review and meta-study synthesis of qualitative studies evaluating facilitators and barriers to participation in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev 2016;25:907-17. [Crossref] [PubMed]
- Javanparast S, Ward PR, Carter SM, et al. Barriers to and facilitators of colorectal cancer screening in different population subgroups in Adelaide, South Australia. Med J Aust 2012;196:521-3. [Crossref] [PubMed]
- Severino G, Wilson C, Turnbull D, et al. Attitudes towards and beliefs about colorectal cancer and screening using the faecal occult blood test within the Italian-Australian community. Asian Pac J Cancer Prev 2009;10:387-94. [PubMed]
- Goel V, Gray R, Chart P, et al. Perspectives on colorectal cancer screening: a focus group study. Health Expect 2004;7:51-60. [Crossref] [PubMed]
- Ekberg M, Callender M, Hamer H, et al. Exploring the decision to participate in the National Health Service Bowel Cancer Screening Programme. Eur J Cancer Prev 2014;23:391-7. [Crossref] [PubMed]
- Griffith KA, Passmore SR, Smith D, et al. African Americans with a family history of colorectal cancer: barriers and facilitators to screening. Oncol Nurs Forum 2012;39:299-306. [Crossref] [PubMed]
- Radecki Breitkopf C, Asiedu GB, Egginton J, et al. An investigation of the colorectal cancer experience and receptivity to family-based cancer prevention programs. Support Care Cancer 2014;22:2517-25. [Crossref] [PubMed]
- Bastani R, Gallardo NV, Maxwell AE. Barriers to colorectal cancer screening among ethnically diverse high- and average-risk individuals. J Psychosoc Oncol 2001;19:65-84. [Crossref]
- Madlensky L, Esplen MJ, Goel V. Reasons given by relatives of colorectal cancer patients for not undergoing screening. Prev Med 2004;39:643-8. [Crossref] [PubMed]
- Rawl SM, Menon U, Champion VL, et al. Colorectal cancer screening beliefs. Focus groups with first-degree relatives. Cancer Pract 2000;8:32-7. [Crossref] [PubMed]
- Pornet C, Denis B, Perrin P, et al. Predictors of adherence to repeat fecal occult blood test in a population-based colorectal cancer screening program. Br J Cancer 2014;111:2152-5. [Crossref] [PubMed]
- Chua AH, Koh GC. Does patient education and recommendation result in increased uptake of colorectal cancer screening using the fecal occult blood test? Ann Acad Med Singapore 2014;43:517-8. [PubMed]
- Ingrand I, Dujoncquoy S, Migeot V, et al. Interactions among physicians, patients, and first-degree relatives in the familial screening of colorectal cancer in France. Patient Prefer Adherence 2008;2:47-55. [PubMed]
- Stermer T, Hodgson S, Kavalier F, et al. Patients' and professionals' opinions of services for people at an increased risk of colorectal cancer: an exploratory qualitative study. Fam Cancer 2004;3:49-53. [Crossref] [PubMed]
- Ingrand I, Dujoncquoy S, Beauchant M, et al. General practitioner and specialist views on colonoscopic screening of first-degree relatives of colorectal cancer patients. Cancer Epidemiol 2009;33:223-30. [Crossref] [PubMed]
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19-32. [Crossref]
- Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. [Crossref] [PubMed]
- Colquhoun HL, Levac D, O'Brien KK, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol 2014;67:1291-4. [Crossref] [PubMed]
- Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team's experience with Arksey and O'Malley's framework. BMC Med Res Methodol 2013;13:48. [Crossref] [PubMed]
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. [Crossref]
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. [Crossref] [PubMed]
- Wong MC, Ching JY, Chiu HM, et al. Risk of colorectal neoplasia in individuals with self-reported family history: a prospective colonoscopy study from 16 asia-pacific regions. Am J Gastroenterol 2016;111:1621-9. [Crossref] [PubMed]
- Wong RK, Wong ML, Chan YH, et al. Gender differences in predictors of colorectal cancer screening uptake: a national cross sectional study based on the health belief model. BMC Public Health 2013;13:677. [Crossref] [PubMed]
- Senore C, Ederle A, Fantin A, et al. Acceptability and side-effects of colonoscopy and sigmoidoscopy in a screening setting. J Med Screen 2011;18:128-34. [Crossref] [PubMed]
- Niv Y, Bogolavski I, Ilani S, et al. Impact of colonoscopy on quality of life. Eur J Gastroenterol Hepatol 2012;24:781-6. [Crossref] [PubMed]
- Ghevariya V, Duddempudi S, Ghevariya N, et al. Barriers to screening colonoscopy in an urban population: a study to help focus further efforts to attain full compliance. Int J Colorectal Dis 2013;28:1497-503. [Crossref] [PubMed]
- Lee YC, Wang HP, Chiu HM, et al. Factors determining post-colonoscopy abdominal pain: prospective study of screening colonoscopy in 1000 subjects. J Gastroenterol Hepatol 2006;21:1575-80. [Crossref] [PubMed]
- Van Ham I, Verhoeven AA, Groenier KH, et al. Job satisfaction among general practitioners: a systematic literature review. Eur J Gen Pract 2006;12:174-80. [Crossref] [PubMed]
- Janus K, Amelung VE, Gaitanides M, et al. German physicians "on strike"--shedding light on the roots of physician dissatisfaction. Health Policy 2007;82:357-65. [Crossref] [PubMed]
- Sahota IS, Kostaras X, Hagen NA. Improving access to cancer guidelines: feedback from health care professionals. Curr Oncol 2015;22:392-8. [Crossref] [PubMed]