Disparities in major surgery for esophagogastric cancer among hospitals by case volume
Introduction
Both short- and long-term outcomes following major oncologic surgery are dependent on a number of factors, including some related to the patient (comorbidities, performance status) and the cancer (stage, grade, resectability). For patients with esophageal and gastric cancer, surgery is often part of the treatment algorithm (1,2). Esophageal resection and total gastrectomy represent two major oncologic procedures that can be associated with considerable morbidity (3,4). Thus, the decision to offer surgery as part of multimodal treatment is often made carefully, balancing the morbidity of the procedure with the expected benefits (5,6).
While the decision to offer and undergo surgery ultimately lies with the patient and the treatment team, other factors extraneous to the patient and the disease have also been shown to influence short-term post-surgical outcomes [such as hospital length of stay (LOS) and 30-day mortality] as well as long-term overall survival (OS). These factors include the experience of the treatment facility and its care team members. Undergoing major surgical procedures, including various oncologic procedures, at high volume centers has been repeatedly associated with superior short- and long-term outcomes (7,8). Performance of major oncologic procedures at high volume centers specifically for esophageal and gastric cancer has similarly been associated with superior outcomes (9-12).
Health care disparities related to patient demographics, including race and socioeconomic status, have also been shown affect access to care and outcomes. This has not only been characterized for various surgical interventions, but also to other treatment modalities such as access to screening, chemotherapy, and radiation therapy (13-15). Specific racial and socioeconomic health disparities have been identified for patients with esophageal or gastric cancer and were associated with poorer outcomes (16,17). Disparities in general and specifically to surgical treatments have become increasingly relevant to health care.
The interaction between treatment center experience as measured by case volume for esophagectomy/total gastrectomy and health care disparities has not been investigated. This represents a major gap in the existing literature because although case volume has been shown to correlate with superior outcomes, the association of treatment facility (based on case volume) with health care disparities is unknown. A better understanding of this association is needed because health care disparities represent a significant, confounding factor that may influence the outcomes achieved at high volume centers. Thus, the purposes of this study were to identify racial and/or socioeconomic disparities among centers performing major surgery for esophageal or gastric cancer, stratified by case volume, and determine the association of these disparities with long-term OS.
Methods
Patients
This retrospective study was deemed exempt from institutional review. Patients were selected from the American College of Surgeons (ACS) National Cancer Data Base (NCDB) from 2004 to 2013 using the Participant User Files (PUF) for both Esophageal and Gastric Cancer. This database, in general, has been shown to comprise approximately 70% of newly diagnosed cancer cases identified in the United States (18). Data were collected on patients with adenocarcinoma histologies using ICD-O-3 (International Classification of Diseases for Oncology, 3rd edition) codes 8140-8148, 8200-8239, 8260-8263, 8480-8496, 8500-8503, and 8560-8573. Patients with squamous cell histology for esophageal cancer were identified using ICD-O-3 codes 8070-8074. Other histologies and patients with benign disease or carcinoma in situ were excluded from the analysis. As this study utilized a nationwide, de-identified database, it was deemed exempt from the Institutional Review Board.
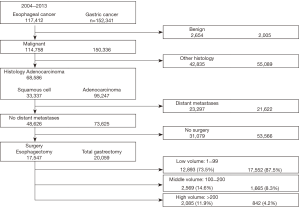
For patients in the Esophageal PUF, major surgical resection was defined as partial esophagectomy, total esophagectomy, and esophagogastrectomy. For patients in the Gastric PUF, only patients who underwent total gastrectomy were included. Patients who had partial, distal, or subtotal gastrectomies were excluded from the analysis. For either malignancy, patients who underwent local procedures, including endoscopic resection or ablation, were excluded. Figure 1 summarizes the sequential inclusion and exclusion criteria used in this study.
Patient factors included age, gender, race, ethnicity, insurance status, income, education, geographic setting, treatment facility type, and distance to treating facility. The NCDB uses the Charlson-Deyo (CD) comorbidity score as a measure of comorbid conditions, which was also included. Treatment centers were divided based on the total number of cases performed during this 10-year time period and were stratified into three groups: low volume [1–99], middle [100–200], and high [>200], which were then compared. Pathologic variables included the primary tumor location for the esophagus (upper, middle, lower third), grade, pathologic stage, and margin status. Post-operative outcomes included hospital LOS, readmission rate, and 30- and 90-day mortality. Other treatment modalities were included, such as chemotherapy and radiation (both neoadjuvant and adjuvant).
Statistical analysis
Patient characteristics were reported by treating facility volume (low, middle, high) using the mean and standard deviation for continuous data, and as frequencies and relative frequencies for categorical data. Comparisons among the facilities were made using Kruskal-Wallis and Pearson chi-square tests for continuous and categorical variables, respectively.
OS was summarized by treating facility volume using standard Kaplan-Meier methods, whereby estimates of median OS and 3- and 5-year OS rates were obtained with 95% confidence intervals (CI). Comparisons were made using the log-rank test. A multivariable analysis was conducted within each facility volume strata using Cox regression; where variables included in the models were obtained using the backwards selection method (alpha-exit of 0.1). All models were fit using Firth’s method. Hazard ratios (HRs) with corresponding 95% CI were obtained from model estimates.
No power analysis was performed as this was an observational study based on a preset national database. All analyses were conducted in SAS v9.4 (Cary, NC, USA) at a nominal significance level of 0.05 and using a Holm-Bonferroni adjustment to control the family-wise error-rate for each set of analyses. Therefore, P values less than 0.05 denote statistically significant associations.
Results
Esophageal cancer and esophagectomy at low, middle and high volume centers
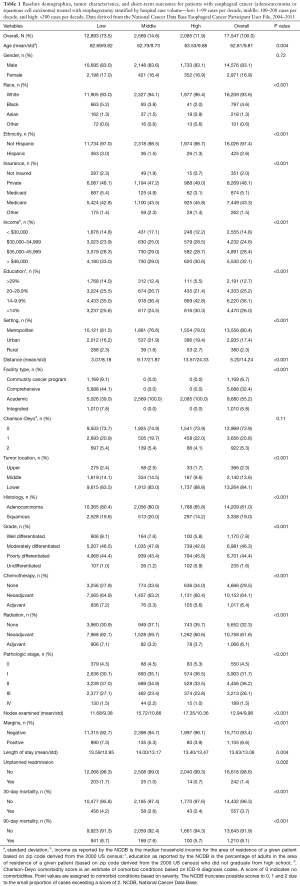
For patients with esophageal cancer, a total of 17,547 patients met the inclusion criteria; 73.5% (12,893) were in the low volume group, 14.6% (2,569) in the middle volume group, and 11.9% (2,085) in the high volume (Figure 1). Table 1 shows the baseline characteristics among these groups. Patients treated at the low volume centers had higher proportions of racial and ethnic minorities, uninsured, and lower education compared to patients treated at the middle and high volume centers. Patients treated at middle and high volume centers traveled further distances and went to only academic centers. Importantly, there was no statistically significant difference in the comorbid status (as indicated by the CD score) among patients treated at any of the three centers (P=0.11).
Full table
Patients treated at low and middle volume centers had higher proportions of squamous cell carcinoma than those at high volume centers. Although differences in pathological stage among the treatment centers reached statistical significance, these differences were no more than approximately 5% for any given stage.
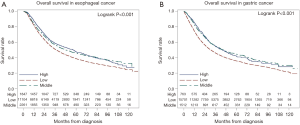
In further comparing these respective groups, those treated at high volume centers had superior short term outcomes: a higher mean number of lymph nodes examined in the resection specimen (11.7, 15.7, and 17.4, for low, middle, and high groups, respectively, P<0.001), lower rates of positive margins (7.3%, 5.3%, 3.9%, P=0.002), shorter mean LOS (10, 10, 9 days, P=0.004), lower unplanned readmission (1.7%, 1.0%, 0.7%, P=0.002), and lower 30-day mortality (4.2%, 2.6%, 2.4%, P<0.001). The median OS was 37.4, 48.4 and 56.9 months, respectively (P<0.001), consistent with previous studies. The median follow-up for the entire cohort was 55.2 months (range, 0.0–130.7 months). The Kaplan Meier curves for patients with esophageal cancer stratified by case volume are shown in Figure 2A.
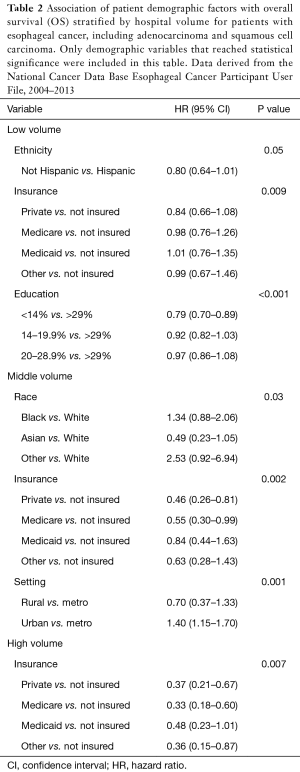
On multivariable analysis of all factors (demographic and pathologic variables in Table 1) for patients treated with esophagectomy, a greater number of disparate factors were identified in the low and middle volume centers compared to the high volume centers. Table 2 summarizes the results for the demographic factors that were found to be significant with OS by hospital volume. These factors were independently associated with OS after analysis with all patient variables, including pathologic and post-operative factors. Among low volume centers, statistically significant disparate factors associated with poorer OS included ethnicity, insurance status, and education. Among middle volume centers, disparate factors included race, insurance status, and hospital setting. And among high volume centers, disparate factors included insurance status only. In general, patients with private insurance and a higher level of education had superior OS compared to uninsured patients and those with a lower level of education. Hispanic ethnicity was associated with worse OS at low volume centers.
Full table
Gastric cancer and total gastrectomy at low, middle and high volume centers
For patients with gastric cancer, a total of 20,059 patients met the inclusion criteria; 87.5% (17,552) were in the low volume group, 8.3% (1,665) in the middle volume group, and 4.2% (842) in the high volume (Figure 1). Table 3 shows the baseline characteristics for these groups. Similar to the esophageal cancer cohort, patients who underwent total gastrectomy at the low volume centers had higher proportions of racial and ethnic minorities, uninsured, and lower education compared to patients treated at the middle and high volume centers. Patients treated at low volume centers also had higher proportions of low income households. Patients treated at middle and high volume centers traveled further distances and went mainly to academic centers. In contrast to patients who underwent esophagectomy, there was a statistically significant difference in the comorbid status (as indicated by the CD score) among patients treated at any of the three centers, with the most ill patients treated at low volume centers. Patients treated at low volume centers also had higher proportions of pathological stage 3/4 disease.
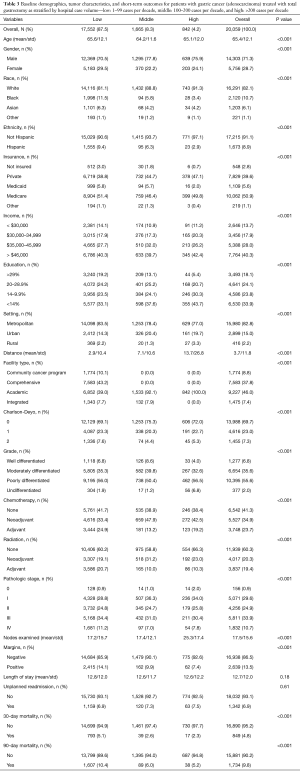
Full table
Similar to the esophageal cancer cohort, those with gastric cancer treated with total gastrectomy at high volume centers had superior short term outcomes: a higher mean number of lymph nodes examined in the resection specimen (17.2, 17.4, and 25.3, for low, middle, and high groups, respectively, P<0.001), lower rates of positive margins (14.1%, 9.9%, 7.4%, P<0.001), and lower 30-day mortality (5.1%, 2.6%, 2.3%, P<0.001). However, there were no differences with respect to post-operative LOS or the unplanned readmission rate. The median OS was 30.8, 45.2 and 43.8 months, respectively (P<0.001), with a similar trend to the esophageal cancer cohort. The median follow-up for the entire cohort was 58.7 months (range, 0.0–129.9 months). The Kaplan Meier curves for patients with gastric cancer stratified by case volume are shown in Figure 2B.
On multivariable analysis for patients treated with total gastrectomy, a greater number of disparate factors was identified in the low and middle volume centers compared to the high volume centers, consistent with the esophagectomy analysis. Table 4 summarizes the results only for the demographic factors that were found to be significant with OS by hospital volume. Among low volume centers, statistically significant disparate factors associated with poorer OS included ethnicity, insurance status, income, and treatment facility type. Among middle volume centers, disparate factors included income only. No racial or socioeconomic disparities were identified among patients treated at the high volume centers. In general, patients with private insurance and a higher level of income had superior OS compared to uninsured patients and those with a lower level of education. Treatment at academic institutions was associated with improved OS at low volume centers.
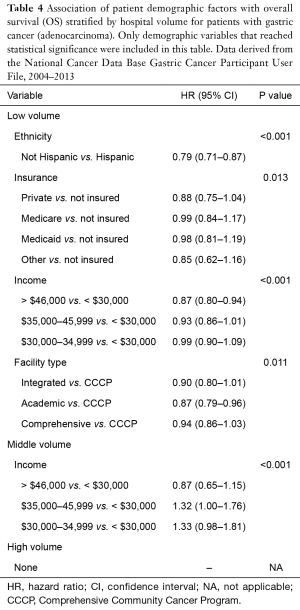
Full table
Discussion
In this study, we characterized the association between health care disparities and outcomes for two major oncologic procedures, esophagectomy and total gastrectomy, stratified by hospital case volume. Each of these issues, (I) disparities in cancer care and (II) the centralization of major surgical procedures based on case volume, has been garnering increasing attention in health care on a global scale (19-22). Disparities in cancer care are pervasive among several cancers, including esophageal and gastric cancers, both in terms of multimodal treatment and for surgery in particular (23-25). Here, we identified important racial and socioeconomic disparities among patients treated with esophagectomy and total gastrectomy. In and of itself, these disparities did not represent novel findings. However, the observations that these disparities varied by treatment center based on case volume, and that these disparities were associated with long-term OS highlight important, novel findings not previously addressed. The use of a large nationwide database to perform this analysis was very appropriate given the large number of patients in both the esophageal and gastric cancer cohorts. The recognition of these disparities contribute to a growing body of evidence that major oncologic procedures should be performed at high volume centers not only because of the benefits to short-term outcomes but also because there are fewer disparities among patients treated at high volume centers.
Importantly, this study confirmed the findings of previous reports characterizing superior short- and long-term outcomes for esophageal cancer (9,10,26,27) and gastric cancer surgery (11,12,28,29) at high volume centers. Access to high volume centers was also limited, reflected by the distance traveled by patients to undergo surgery at the higher volume centers (Tables 1,3). However, the unique findings of this study showed that while both short- and long-term outcomes were superior at high volume centers, these benefits may not be equally appreciated by all patient populations. Health care treatment disparities have been previously identified for patients with either esophageal (16,30) or gastric cancer (17,25,31). This study provided an in-depth analysis demonstrating that health care disparities vary by institution based on case volume for the selected oncologic procedures, with fewer disparate factors present at high volume centers. On multivariable analysis, these disparities were independently associated with OS and may represent areas for health care reform and support centralization of complex surgery at high volume centers.
Another issue related to post-operative outcomes is failure to rescue. This concept applies to patients who have sustained post-operative complications and are unable to recover from them, ultimately progressing to mortality (32,33). A variety of factors has been associated with failure to rescue, including both patient and treatment center characteristics. With regard to the latter, the type of treatment center (academic teaching versus non-teaching), nurse-to-patient ratio, and number of ICU (intensive care unit) beds were associated with failure to rescue (34). Interestingly, health care disparities have also been linked to failure to rescue. In fact, Reames et al. utilized the Medicare Provider Analysis and Review File and the Medicare Denominator File to study major oncologic procedures, including esophagectomy and total gastrectomy (35). The authors showed that patients with lower socioeconomic status had significantly higher rates of failure to rescue.
There have been some studies which have not shown an association between hospital case volume and short-term outcomes. One example was a study performed by LaPar et al. using the 2008 Nationwide Inpatient Sample (NIS) (36). In this study, the relationship between hospital procedure volume of major surgeries and mortality was assessed using adjustments for patient demographics, comorbid disease, and elective procedure status. The authors concluded that hospital procedure volume was not a significant predictor of mortality for the performance of pancreatectomy, AAA (abdominal aortic aneurysm) repair, esophagectomy, or CABG (coronary artery bypass graft). In a more focused study by Kozower et al. examining only esophagectomies again using the NIS, no significant association was demonstrated between in-hospital mortality and procedure volume (37). While the results of these studies differ from our own findings with regard to morbidity, it is necessary to note these studies utilize different databases so that in inherent differences between the NCDB and NIS may have accounted for these differences. Furthermore, whereas our study also examined the effect of these disparities on OS, these prior studies using the NIS were limited to short-term outcomes and did not assess survival.
We recognize that there were important limitations to this study. First, there were inherent limitations to the database. With respect to assigning centers to low, middle, and high volume, there were disproportionate numbers among each of these groups, with the majority of patients receiving treatment at the low volume centers. Thus, there were statistically greater chances of identifying disparities among the low volume centers compared to the middle or high volume centers. To adjust for this limitation, however, we used the Holm-Bonferroni method to minimize statistically significant associations that could be discovered by chance. In addition, there is evidence to suggest that superior outcomes associated with high volume centers were more related to high volume surgeons as opposed to the overall care received at these centers. However, the NCDB does not provide sufficient data to analyze outcomes based on single surgeon volume (38).
With respect to the disparities analysis, although the NCDB captures the majority of cancer cases in the US and allows for a robust statistical analysis, patients in minority populations or those with lower socioeconomic status may be less likely to be treated at the cancer centers participating in the NCDB. Therefore, there may have been a selection bias whereby the sample represented by the NCDB in general was skewed toward Caucasian patients or those with higher socioeconomic status. Another limitation was that although the demographic variables included in the NCDB were quite comprehensive, the categories within each variable were more limiting. For example, the NCDB uses the CD comorbidity score as a measure of patient comorbidities, which is truncated to three values (0 indicates no comorbidities, 1 indicates a single selected comorbidity, and 2 indicates ≥1 of the selected comorbidities). Similarly, the NCDB has defined cutoff values for income and education derived from 2000 US Census data, which were somewhat narrow and may have been outdated in our study population. Other health care disparities, such as the type of insurance plans that certain treatment centers accept, were not available in the database. As an example, treatment centers without an emergency room would be expected to have fewer uninsured or Medicaid patients, representing a bias in the analysis.
In conclusion, this study shows an increasing number of disparate patient factors associated with low and middle volume centers compared to high volume centers for patients treated with esophagectomy or total gastrectomy, and these factors were independently associated with worse OS on multivariable analysis. We showed for the first time that disparities differed among treatment centers based on volume. In addition to the improved short term outcomes and OS, this study further makes the case for performance of esophagectomy and total gastrectomy at high volume centers, where fewer disparities were observed.
Acknowledgements
We thank the Commission on Cancer of the American College of Surgeons for access to the NCDB Participant User File.
Funding: The statistical analysis was supported by Roswell Park Cancer Institute and National Cancer Institute (NCI) grant P30CA016056.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As this study utilized a nationwide, de-identified database, it was deemed exempt from the Institutional Review Board.
References
- National Comprehensive Cancer Network. Rectal Cancer (Version 4.2014). Available online: http://www.nccn.org/professionals/physician_gls/pdf/rectum.pdf
- National Comprehensive Cancer Network. Breast Cancer (Version 1.2015). Available online: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Ben-David K, Sarosi GA, Cendan JC, et al. Decreasing morbidity and mortality in 100 consecutive minimally invasive esophagectomies. Surg Endosc 2012;26:162-7. [Crossref] [PubMed]
- Ben-David K, Tuttle R, Kukar M, et al. Minimally Invasive Esophagectomy Utilizing a Stapled Side-to-Side Anastomosis is Safe in the Western Patient Population. Ann Surg Oncol 2016;23:3056-62. [Crossref] [PubMed]
- Gabriel E, Attwood K, Du W, et al. Association Between Clinically Staged Node-Negative Esophageal Adenocarcinoma and Overall Survival Benefit From Neoadjuvant Chemoradiation. JAMA Surg 2016;151:234-45. [PubMed]
- Gabriel E, Attwood K, Shah R, et al. Novel Calculator to Estimate Overall Survival Benefit from Neoadjuvant Chemoradiation in Patients with Esophageal Adenocarcinoma. J Am Coll Surg 2017;224:884-94.e1. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Allareddy V, Allareddy V, Konety BR. Specificity of procedure volume and in-hospital mortality association. Ann Surg 2007;246:135-9. [Crossref] [PubMed]
- Speicher PJ, Englum BR, Ganapathi AM, et al. Traveling to a High-volume Center is Associated With Improved Survival for Patients With Esophageal Cancer. Ann Surg 2017;265:743-9. [Crossref] [PubMed]
- Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut 2014;63:1393-400. [Crossref] [PubMed]
- Alvino DM, Chang DC, Adler JT, et al. How Far Are Patients Willing to Travel for Gastrectomy? Ann Surg 2017;265:1172-7. [Crossref] [PubMed]
- Yang K, Choi YY, Zhang WH, et al. Strategies to improve treatment outcome in gastric cancer: a retrospective analysis of patients from two high-volume hospitals in Korea and China. Oncotarget 2016;7:44660-75. [PubMed]
- Trinh QD, Sun M, Sammon J, et al. Disparities in access to care at high-volume institutions for uro-oncologic procedures. Cancer 2012;118:4421-6. [Crossref] [PubMed]
- Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol 2003;21:1293-300. [Crossref] [PubMed]
- Hao Y, Landrine H, Jemal A, et al. Race, neighbourhood characteristics and disparities in chemotherapy for colorectal cancer. J Epidemiol Community Health 2011;65:211-7. [Crossref] [PubMed]
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Racial disparities in esophageal cancer survival after surgery. J Surg Oncol 2016;113:659-64. [Crossref] [PubMed]
- de Vries E, Uribe C, Pardo C, et al. Gastric cancer survival and affiliation to health insurance in a middle-income setting. Cancer Epidemiol 2015;39:91-6. [Crossref] [PubMed]
- Winchester DP, Stewart AK, Phillips JL, et al. The national cancer data base: past, present, and future. Ann Surg Oncol 2010;17:4-7. [Crossref] [PubMed]
- von Dercks N, Gockel I, Mehdorn M, et al. Economic aspects of oncological esophageal surgery: Centralization is essential. Chirurg 2017;88:62-9. [Crossref] [PubMed]
- Pasquer A, Renaud F, Hec F, et al. Is Centralization Needed for Esophageal and Gastric Cancer Patients With Low Operative Risk?: A Nationwide Study. Ann Surg 2016;264:823-30. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. Pattern of Postoperative Mortality After Esophageal Cancer Resection According to Center Volume: Results from a Large European Multicenter Study. Ann Surg Oncol 2015;22:2615-23. [Crossref] [PubMed]
- Mariette C, Taillier G, Van Seuningen I, et al. Factors affecting postoperative course and survival after en bloc resection for esophageal carcinoma. Ann Thorac Surg 2004;78:1177-83. [Crossref] [PubMed]
- Gabriel E, Thirunavukarasu P, Al-Sukhni E, et al. National disparities in minimally invasive surgery for rectal cancer. Surg Endosc 2016;30:1060-7. [Crossref] [PubMed]
- Gabriel E, Thirunavukarasu P, Attwood K, et al. National disparities in minimally invasive surgery for pancreatic tumors. Surg Endosc 2017;31:398-409. [Crossref] [PubMed]
- Glenn JA, Turaga KK, Gamblin TC, et al. Minimally invasive gastrectomy for cancer: current utilization in US academic medical centers. Surg Endosc 2015;29:3768-75. [Crossref] [PubMed]
- Markar SR, Karthikesalingam A, Thrumurthy S, et al. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000-2011. J Gastrointest Surg 2012;16:1055-63. [Crossref] [PubMed]
- Wouters MW, Gooiker GA, van Sandick JW, et al. The volume-outcome relation in the surgical treatment of esophageal cancer: a systematic review and meta-analysis. Cancer 2012;118:1754-63. [Crossref] [PubMed]
- Ihemelandu C, Zheng C, Hall E, et al. Multimorbidity and access to major cancer surgery at high-volume hospitals in a regionalized era. Am J Surg 2016;211:697-702. [Crossref] [PubMed]
- Enzinger PC, Benedetti JK, Meyerhardt JA, et al. Impact of hospital volume on recurrence and survival after surgery for gastric cancer. Ann Surg 2007;245:426-34. [Crossref] [PubMed]
- Thirunavukarasu P, Gabriel E, Attwood K, et al. Nationwide analysis of short-term surgical outcomes of minimally invasive esophagectomy for malignancy. Int J Surg 2016;25:69-75. [Crossref] [PubMed]
- Luyimbazi D, Nelson RA, Choi AH, et al. Estimates of conditional survival in gastric cancer reveal a reduction of racial disparities with long-term follow-up. J Gastrointest Surg 2015;19:251-7. [Crossref] [PubMed]
- Weledji EP, Verla V. Failure to rescue patients from early critical complications of oesophagogastric cancer surgery. Ann Med Surg (Lond) 2016;7:34-41. [Crossref] [PubMed]
- Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care 2011;49:1076-81. [Crossref] [PubMed]
- Sheetz KH, Dimick JB, Ghaferi AA. Impact of Hospital Characteristics on Failure to Rescue Following Major Surgery. Ann Surg 2016;263:692-7. [Crossref] [PubMed]
- Reames BN, Birkmeyer NJ, Dimick JB, et al. Socioeconomic disparities in mortality after cancer surgery: failure to rescue. JAMA Surg 2014;149:475-81. [Crossref] [PubMed]
- LaPar DJ, Kron IL, Jones DR, et al. Hospital procedure volume should not be used as a measure of surgical quality. Ann Surg 2012;256:606-15. [Crossref] [PubMed]
- Kozower BD, Stukenborg GJ. Hospital esophageal cancer resection volume does not predict patient mortality risk. Ann Thorac Surg 2012;93:1690-6; discussion 6-8.
- Lee HH, Son SY, Lee JH, et al. Surgeon's Experience Overrides the Effect of Hospital Volume for Postoperative Outcomes of Laparoscopic Surgery in Gastric Cancer: Multi-institutional Study. Ann Surg Oncol 2017;24:1010-7. [Crossref] [PubMed]


